Cadmium (Cd) is released into the environment by natural sources, agriculture and manifold industrial uses (Hartwig 2010). Cd pollution is well known as one of the most serious environmental problems worldwide due to the rapid expansion of industrialization and the heavy use of chemical fertilizers, pesticides and herbicides in agriculture (Zacchini et al. 2009). Cd can be absorbed and accumulated in plant tissues where roots are the primary site of accumulation (Vitória et al. 2001, Ge et al. 2012). Heavy metal tolerance in higher plants is the result of different processes which prevent excess of heavy metals in the cytoplasm and organelles. Many reports have addressed Cd toxicity in plants such as disturbance of mitosis (Xu et al. 2009, Zhang et al. 2009, Shi et al. 2014), toxicity on nucleoli (Zhang et al. 2009, Zou et al. 2012, Qin et al. 2013a) causing inhibition of root and shoot growth (Ge et al. 2012, Jiao et al. 2012). Although Cd toxicity and plant tolerance mechanisms have been widely discussed (Sanità di Toppi et al. 2007, Choppala et al. 2014, Upadhyay 2014; Asgher et al. 2015), Cd tolerance strategies on cellular and molecular level have not been fully explained yet. Therefore, it is very important to elucidate Cd toxicity to plants and the possible mechanisms.
Higher plants provide a useful genetic system for screening and monitoring environmental pollutants. Allium cepa, Vicia faba, Arabidopsis thaliana, Hordeum vulgare etc. are most widely used in general toxicity studies (Fiskesjö 1985, Sirri et al. 2008, Liu et al. 1995, Liman et al. 2010, Özkara et al. 2011). Among them, H. vulgare is well known as a biological model and a useful bioindicator for the detection of heavy metal pollution (Özkara et al. 2011). Cd toxic effects on nucleoli in root tip cells of H. vulgare by means of silver staining, immunofluorescence staining and western blotting were investigated in order to further understand the Cd toxic mechanism. The data obtained here are useful to understand better the mechanisms of Cd-induced cell toxicity.
Materials and methods
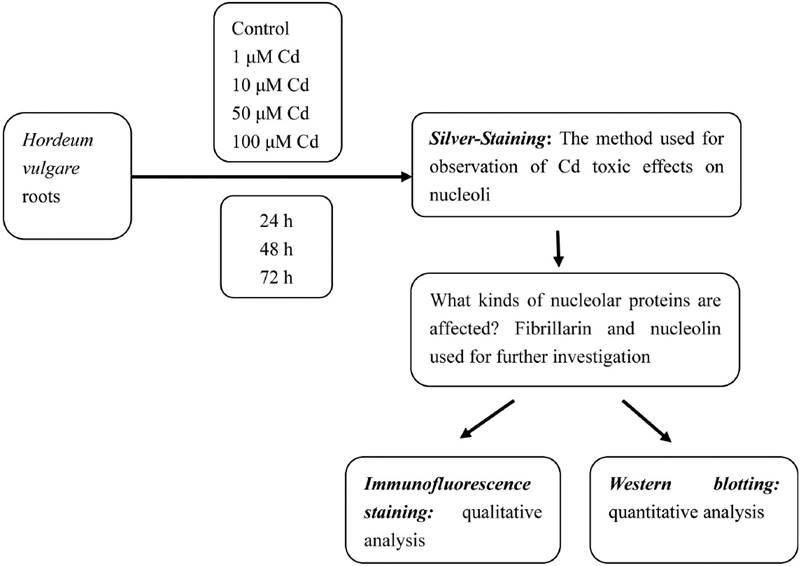
The experimental procedure of this work are outlined in Figure 1.
Culture conditions and cadmium treatment. Healthy equally-sized seeds of Hordeum vulgare were collected and soaked in distilled water for 24 h before starting the experiments. After the seeds were germinated in moistened gauze in the dark at 23 ºC for 12 h, they grew in plastic containers at 23 ºC for 24 h, producing roots reaching about 0.6 cm length. Then the seedlings were exposed to different concentrations of Cd solutions (1, 10, 50 and 100 µM) for 24, 48, and 72 h. Cadmium chloride (CdCl2) was used in the present investigation. The solutions were adjusted to pH 5.5, aerated by pump, which connected the containers with pump lines. Control seedlings were grown in distilled water alone. The solutions for the experiment were renewed regularly every 24 h. All treatments were done by triplicates.
Silver-Staining. Twenty root tips of Hordeum vulgare treated with 1, 10, 50 and 100 µM Cd were cut every 24 h till 72 h. For the observation of changes in nucleoli, ten root tips were cut, were fixed in 3 parts 95 % ethanol:2 parts acetic acid for 4 to 5 h and hydrolysed in 1M hydrochloric acid, 95 % ethanol and acetic acid (5:3:2, v/v) for 4-5 min at 60 ºC, followed by squashing in 45 % acetic acid, dried in an oven at 35 ºC , and after 2 days stained with 50 % silver nitrate (w/v) (Liu & Jiang, 1991).
Immunofluorescence Staining. In the present investigation, 50 µM Cd was found to have obviously toxic effect on the root growth and root tip cells of Hordeum vulgare. In order to observe the obviously toxic effects of Cd on nucleolin and fibrillarin, 50 µM Cd was chosen. Meristematic zones were cut in root tips of untreated H. vulgare or treated with 50 µM Cd for 72 h were cut and fixed with 4 % (w/v) paraformaldehyde in phosphate-buffered saline (PBS, pH 7.0) for 2 h in darkness at room temperature and then they were washed with the same buffer. Meristematic cells were digested with a mixture of 2.5 % cellulase and 2.5 % pectolase at 37 °C and then washed in PBS for three times. They were squashed on slides and extracted in freshly prepared 1 % (v/v) Triton X-100 in PBS when slides dried. After three washings in PBS, the cells were subsequently incubated with mouse primary antibodies respectively against fibrillarin and nucleolin for 1 h at 37 °C or at 4 °C overnight in a moist, sealed chamber. After washing (3 × 10 min) in PBS, the cells were incubated with secondary antibodies for detection of the primary antibodies for 45 min in darkness at 37 °C. After repeated washing in PBS, nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI, Sigma) at a final concentration of 1 µg per 1 ml for 15 min at room temperature. After washing (3 × 10 min) in PBS, the cells were mounted in antifade mounting medium. The slides were stored at 4 °C in the dark until observed. The immunofluorescent specimens were examined under a fluorescence microscope (Nikon, HB-10101AF) with the digital camera Pixera Pro 600CL, using violet (355–425 nm) and blue (450–490 nm) specific filters for proteins and nuclei respectively. Photographs were taken and images were processed with Adobe Photoshop 7.0.
Antibodies used in this study were as follows: 1) Fibrillarin. - primary antibody: a mousemonoclonal antibody to fibrillarin (Santa, SC-81273) at dilution 1:100; secondary antibody: FITC-conjugated goat anti-mouse IgGs (Sigma, F9137) at dilution 1:50. FITC was used for the detection of signal. 2) Nucleolin/C23. - primary antibody: a mouse monoclonal antibody to nucleolin (Santa, SC-8031) at dilution 1:100; secondary antibody: FITC-conjugated goat anti-mouse IgGs (Sigma, T5393) at dilution 1:50. TRITC was used for the detection of signal (Qin et al. 2013a).
Western Blotting. Root tips from control and seedlings treated with Cd for 72 h were homogenized respectively in a pestle and mortar with liquid nitrogen and then the samples were solubilized with chilled extraction buffer (50 mM Tris–HCl (pH 7.8), 10 mM MgCl2, 20 mM β-mercaptoethanol, 1.0 mM EDTA, 8 % glycerol) adding protease inhibitor cocktail set VI (Merck, 539133). After vortexing for 1 min at room temperature, the homogenates were kept on ice for 30 min, and then centrifuged at 12,000 rpm at 4 °C for 5 min. The mixture of supernatant and 1 × laemmli buffer (62.5 mM Tris –HCl (pH 6.8), 5 % β-mercaptoethanol, 2 % SDS, 10 % glycerol, 0.001 % bromophenol blue) was boiled at 100 °C for 5 min (Laemmli 1970), determined the protein content according to the Bradford method (Bradford 1976) and then subjected to 12 % SDSPAGE electrophoresis and the separated proteins were wet blotted onto 0.45 µm PVDF transfer membrane (Millipore, IPVH00010) at 4 °C. Blots were blocked for 2 h with 5 % (w/v) non-fat milk in TBST buffer at room temperature with shaking. Primary antibody mentioned above against the indicated protein was diluted in TBST buffer (B23, 1:4000; Fibrillarin, 1:4000; C23, 1:3500). Anti-β actin monoclonal antibody (Abmart, P30002) was used for the internal control. The soaked PVDF membrane was subsequently incubated with primary antibody for 2.5 h at room temperature on the rocker platform and then washed with TBST buffer two times for 10 min each and TBS buffer one time for 10 min. The HRPconjugated secondary antibody (Promega, W4021) diluted 1:7000 in TBST buffer was added for 2 h. After another three washes, the blots were detected using the ECL technique (Millipore, WBKL S0100) and exposed to the X-ray film. The bands in the film were determined using the software “Quantity One” (Bio-Rad). During the experiment, care was taken to prevent membrane from drying (Qin et al. 2013a).
Statistical Analysis. Each treatment was triplicated for statistical validity. Results here were analyzed with standard statistical software (SPSS) and SigmaPlot 8.0 using mean ± standard error (SE). Any differences between treatments were determined using one-way analysis of variance (ANOVA). For equality of averages the t-test was applied. Results were considered statistically significant at P < 0.05.
Results
Effects of Cd on nucleolus morphology. Generally, there are 1 to 2 dark-brown nucleoli in the nucleus of Hordeum vulgare (Figure 2A). The toxic effects of Cd on nucleoli varied with the concentration and the treatment time used. Three phenomena were observed under Cd stress. Firstly, at low concentration (10 µM Cd) for 48 h, nucleoli were irregular in morphology (Figure 2B–C). Big swollen nucleolus occupied large areas of the nucleus was also noted (Figure 2D). Secondly, some tiny silver-stained particles together with the nucleolus were observed in the nucleus of some root tip cells exposed to 50 µM Cd for 24 h (Figure 2E). Thirdly, the particles were accumulated and leached out from the nucleus to the cytoplasm after 50 µM Cd for 48 h (Figure 2F). The amount of this particulate material increased progressively in cytoplasm (Figure 2G) and nearly occupied the whole cytoplasm when the Cd concentration increased to 100 µM Cd (Figure 2H–I).
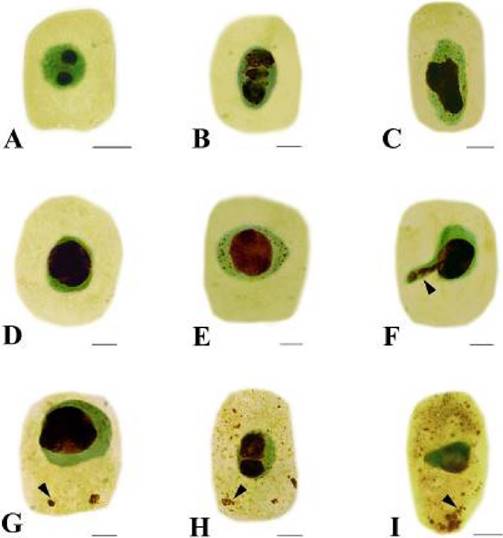
Figure 2 Effects of Cd on nucleoli in root tip cells of Hordeum vulgare. A. Control cell. B–C. Showing irregular nucleoli. B. 10 µM Cd; 48 h. C. 50 µM Cd; 24 h. D. Showing big swollen nucleolus, which occupied large areas of the nucleus (50 µM Cd; 24 h). E. Showing silver-stained particles together with the nucleolus scattered in the nucleus (50 µM Cd; 24 h). F. Showing silver-stained materials extruded from the nucleus into the cytoplasm (50 µM Cd; 48 h). G. Showing the nucleolus materials increased in the cytoplasm with prolonged treatment time (50 µM Cd; 72 h). H–I. Showing more and more silver-stained particles scattered in the cytoplasm with increasing Cd concentration (H. 100 µM Cd; 24 h; I. 100 µM Cd; 48 h). Scale bars = 10 µm. Arrowheads show nucleolus materials extruded from the nucleus into the cytoplasm.
Effects of Cd on nucleoproteins. Immunofluorescence localizations of nucleolin and fibrillarin were performed in the present study. The antibodies used for the detection of these proteins may produce positive reactions with the NPs above. There were toxic effects of Cd on the NPs in the root tip cells of Hordeum vulgare after 50 µM Cd treatment when compared with control cells.
Nucleolin was marked with FITC, producing green fluorescent signal under confocal microscopy. Data here showed that a few green immunofluorescent spots of nucleolin were scattered in nucleoli of control cells (Figure 3A1–A3). Exposure of cells to 50 µM Cd for 24 h, showed that nucleolin migrated from the nucleolus to the nucleoplasm (Figure 3B). After 50 µM Cd treatment for 48 h, nucleolin signal was observed in the nucleoplasm (Figure 3C). More nucleolin signals were noted in the nucleoplasm and cytoplasm (Figure 3D).
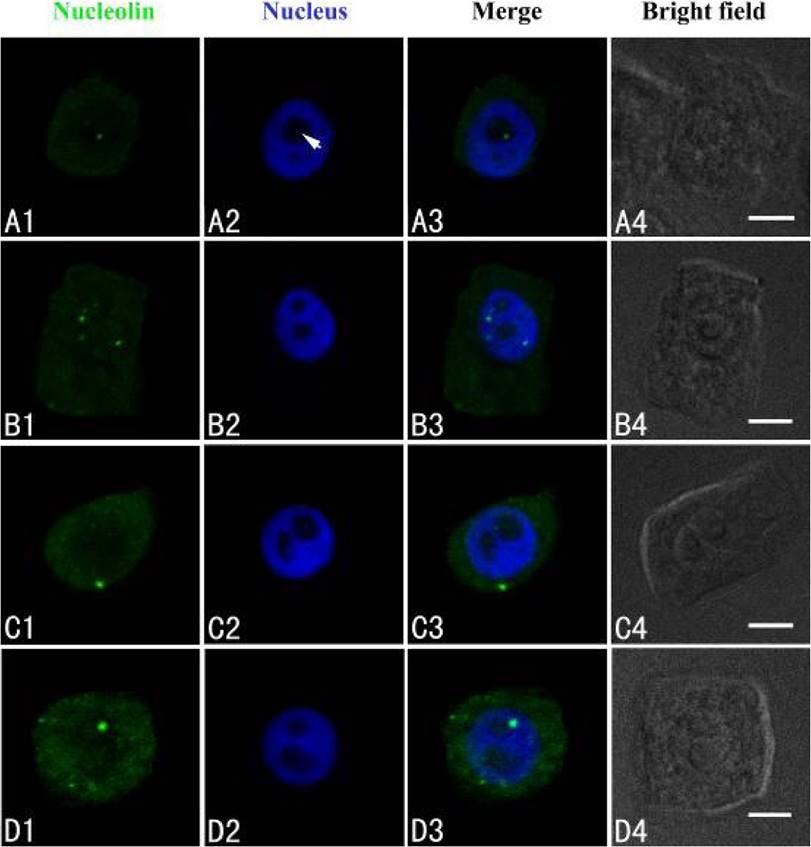
Figure 3 Simultaneous detection of nucleolin after incubation with primary anti-nucleolin antibody and secondary antibody conjugated with FITC (green), and of DNA after incubation with DAPI (blue) in the same single optical section using confocal microscopy. A1, B1, C1 and D1: Nucleolin detection; A2, B2, C2 and D2: DNA detection; A3, B3, C3 and D3: Merged image of “nucleolin detection” and “DNA detection”; A4, B4, C4 and D4: Bright field image. A1, A2, A3 and A4: Nucleolin in nucleolus of control cells; B1, B2, B3 and B4: The migration of nucleolin from the nucleolus to the nucleoplasm in cells treated with 50 µM Cd for 24 h; C1, C2, C3 and C4: Nucleolin in the cytoplasm of cells treated with 50 µM Cd for 48 h; D1, D2, D3 and D4: The signal intensity increased in the cytoplasm of cells treated with 50 µM Cd for 72 h. Scale bars = 10 µm. Arrowhead shows nucleolus.
The intracellular distribution of fibrillarin was similar to nucleolin using confocal laser scanning microscopy. Fibrillarin was localized exclusively in the nucleoli of control cells (Figure 4A1–A3). In the cells exposed to 50 µM Cd for 24 h, disordered immunofluorescence patterns of fibrillarin were observed, revealing that fibrillarin migrated from the nucleoli to the nucleoplasm or on the way to cytoplasm (Figure 4B1–B3). After 48 h of treatment, it appeared in the cytoplasm (Figure 4C1–C3), and in some cells, it was present only in the cytoplasm. The signal intensity increased in the cytoplasm in cells exposed to Cd for 72 h (Figure 4D1–D3).

Figure 4 Simultaneous detection of fibrillarin after incubation with primary anti-fibrillarin antibody and secondary antibody conjugated with FITC (green), and of DNA after incubation with DAPI (blue) in the same single optical section using confocal microscopy. A1, B1, C1 and D1: Fibrillarin detection; A2, B2, C2 and D2: DNA detection; A3, B3, C3 and D3: Merged image of “fibrillarin detection” and “DNA detection”; A4, B4, C4 and D4: Bright field image. A1, A2, A3 and A4: Fibrillarin in the nucleolus of control cells; B1, B2, B3 and B4: Migration of fibrillarin from the nucleolus to the nucleoplasm or the cytoplasm in the cells treated with 50 µM Cd for 24 h; C1, C2, C3 and C4: Fibrillarin in the cytoplasm of the treated cells for 48 h. D1, D2, D3 and D4: The signal intensity increased in the cytoplasm after the treatment with Cd for 72 h. Scale bars = 10 µm. Arrowhead shows nucleolus.
Expression of the two major nucleoproteins. Anti-β actin was used as the internal control (Figure 5A). The contents of nucleolin and fibrillarinin root tip cells of Hordeum vulgare exposed to 50 µM Cd for 72 h were analyzed by western blotting, raised with specific antibodies. The evidences indicated that levels of the two examined proteins augmented significantly (P < 0.05) in comparison with control plants (Figure 5), consistent with the results obtained by indirect immunofluorescent microscopy.
Discussion
It has been well known that plant roots are one of the most sensitive organs to environmental stresses (Qin et al. 2013a). The roots are the sole organ penetrating the soil, directly contacting with toxic metals. It is expected that they are the first to undergo toxicity and develop the responding symptoms. Therefore, the evaluation of action mechanisms of Cd toxic to plant root tip cells in the present investigation is very important.
The evidence has demonstrated that nucleoli are the ribosome factory of the cells and plays several crucial functions in the nucleus. Nucleolus contains a set of acidic, nonhistone proteins that have a high affinity for silver ions and can be selectively visualized by silver staining methods (Sirri et al. 2008). In 1983, Fiskesjö firstly found that Al could induce nucleolar material extruded from the nuclei into the cytoplasm in root cells of Allium cepa exposed to Al using the Feulgen-light green procedure (Fiskesjö 1983). The phenomenon was called as ‘Al-structure’ (Fiskesjö 1983), which was not observed in Allium tests with some ten other metal ions (Fiskesjö 1988). However, changes of argyrophilic proteins in nucleoli can be showed specifically using the silver staining method. Later, the ‘Al-structure’ phenomenon was confirmed by Liu & Jiang (1991) using the silver staining technique for the first time. After that, we found that besides Al, some heavy metals such as Cd, Pb, Cu, Ni, Co, Mg and Hg could also affect nucleoli and induce the phenomenon mentioned above in Allium cepa, A. sativum, Vicia faba and Zea mays (Liu et al. 1995, Jiang & Liu 2000, Liu et al. 2003, 2004, Liu et al. 2009, Qin et al. 2013a, Jiang et al., 2014), but the amounts of extruded materials were less than in the Al treatment. The Cd-induced phenomena observed in this investigation were that some tiny particles containing argyrophilic proteins were scattered in the nucleus of root tip cells and leached out from the nucleus to the cytoplasm (Figure 2). Once the nucleolus was affected, the root growth of Hordeum vulgare was clearly inhibited. These toxic effects of Cd on nucleoli are in agreement with the observations where toxic effects of Cd on nucleoli in root tips of A. sativum (Liu et al. 2003, 2004) and V. faba (Zhang et al. 2009, Qin et al. 2013a) were investigated, but with the difference. There are not so many nucleolar particles scattered in the nuclei and so much nucleolar material released from nuclei into cytoplasm in H. vulgare when compared with Z. mays and V. faba, revealing that Cd toxicity on the nucleoli in root tip cells of A. sativum and V. faba is stronger than those of H. vulgare.
Nucleolus has been known to play important roles in the regulation of many fundamental cellular processes, including cell cycle regulation, apoptosis, telomerase production, RNA processing, monitoring and response to cellular stress (Boisvert et al. 2007). The nucleolus contains several kinds of NPs such as nucleolin and fibrillarin, which are known as the two major and multifunctional NPs, participating in rRNA processing and cell activities. They take key roles in rDNA transcripts and ribosome assembly (Sobol et al. 2006, Ugrinova et al. 2007, Tajrishi et al. 2011, Bhatt et al. 2012). As for what kind of NPs influenced by Cd, there are few reports concerning with them. The evidence from indirect immunofluorescent microscopy demonstrate that fibrillarin and nucleolin are also migrated from the nucleoli to the nucleoplasm or to cytoplasm in the root tip cells of Allium cepa and Hordeum vulgare under Al, Pb and Cu stress, which is consistent with the phenomena obtained from silver staining method. (Qin et al. 2013b, Jiang et al. 2014, Wang et al. 2015). Data here revealed that the two NPs were localized in nucleoli in the untreated root tips of H. vulgare. After exposure to Cd for 24 to 48 h they migrated from the nucleolus to the nucleoplasm or cytoplasm and were over-expressed, which further confirms the phenomenon ‘Al-structure’ named by Fiskesjö (1983, 1990). This increased expression may mirror an enhancement of nucleolar activity which is an important aspect of the cellular/nucleolar response to Cd stress. The observation here supported the findings reported by Qin et al. (2013a) where effects of Cd on nucleoli in Vicia faba root tips were investigated. Nucleolin belongs to the major Ag-NOR protein (Ginisty et al. 1999), while fibrillarin is distinguished from them by its lack of affinity for silver staining (Strauss & Wilson 1990), suggesting that Cd may also have toxic effects on other kinds of NPs besides argyrophilic and acidic NPs. However, more studies are required in this direction. Nucleolin is found in yeasts, plants and mammals and involved in essential processes of ribosome biogenesis, and may interact directly to promote plant growth (Chathoth et al. 2009, Sripinyowanich et al. 2013). Fibrillarin is present both in animal and plant cells. It has a molecular mass of 41 kDa and is required for multiple events leading to rRNA maturation and ribosome subunit assembly (Chen & Huang 2001, Pianta et al. 2011). Pianta et al. (2011) indicated that protein acetylation could control localization of nucleophosmin.
Conclusion
The findings of the present investigation confirm that (1) Cd can affect nucleolus in the root tip cells of Hordeum vulgare, inducing extrusion of silver-stained materials containing argyrophilic proteins from the nucleolus into the cytoplasm. (2) H. vulgare is not sensitive to Cd when compared with Allium sativum and Vicia faba. (3) The nucleolar material contains nucleolin and fibrillarin. (4) Western blotting reveals higher expression of the two major nucleoproteins in Cd-treated roots, which is consistent with the results obtained by indirect immunofluorescence. (5) The findings here supports the discoveries by Fiskesjö (1983) in which nucleolar material in root cells of Allium cepa treated with Al was extruded from the nuclei into the cytoplasm using the Feulgen-light green procedure and our former works concerning with other metals. (6) The results support the findings by Qin et al. (2013a) in which they observed that Cd could affect nucleolin and fibrillarin in the root tip cells of V. faba after the treatment with Cd, inducing the two nucleolarproteins extruded from the nucleolus into the cytoplasm for the first time. (7) H. vulgare can be well known as a useful bioindicator for the detection of heavy metal pollution. The data obtained here can provide valuable information for monitoring and forecast early effects of exposure to Cd in polluted environments.











 nueva página del texto (beta)
nueva página del texto (beta)