The fossil record is a unique source of information that expands our vision of past life. However, its recognition does not mean that it is fully understood nor properly used to address evolutionary aspects beyond the mere documentation of organisms in the past. The somewhat passive attitude towards fossils in Mexico could be explained by their limited economic benefits (except for their use in hydrocarbon exploration), resulting in slow scientific development, fostered only by a cultural and intellectual interest (Weber & Cevallos-Ferriz 1994, Cevallos-Ferriz et al. 2012, Villanueva-Amadoz et al. 2014).
Fossils were known in Mexico before the colonial period. Fossil fish have been found in stuccoes of pyramids in southern Mexico (Carreño & Montellano-Ballesteros 2005, Gío-Argáez & Rodríguez Arévalo 2003) and, during colonial times, Mexican fossils were sent to Spain, England, Germany, and France for study (Carreño & Montellano-Ballesteros 2005, Gío-Argáez & Rodríguez Arévalo 2003). Thus, in a way, knowledge of Mexican fossils began abroad. In the late 1800s, Mexican researchers were mainly describing vertebrates (notably Pleistocene proboscideans and mammoths) and invertebrates, which marked the beginning of paleontological studies in Mexico, with samples from Puebla, Sonora, and Baja California states. Microfossils, especially those related to the study of oil and coal deposits (foraminifera), prompted paleontology to be applied to stratigraphy (Carreño & Montellano-Ballesteros 2005, Gío-Argáez & Rodríguez Arévalo 2003). The study of fossil plants has been less emphasized since those times compared to that of vertebrates and invertebrates.
Few reports of fossil plants exist before the 1970s (Weber & Cevallos-Ferriz 1994, Villanueva-Amadoz et al. 2014). The first publication comes from Felix & Lenk (1888-1989), who reported wood of legumes from presumably Cretaceous strata from Tlacolula, Oaxaca. Although the described outcrops have not been relocated, Centeno-González et al. (2021) found a Cretaceous fruit that resembles their Leguminosae, in a distant locality in Coahuila. Subsequently, Wieland (1914) visited Oaxaca and found abundant and meaningful outcrops with Jurassic plants that he described in detail (Wieland 1913). In the 1960s and early 1970s, Delevoryas and his students (e.g., Delevoryas 1968, Delevoryas & Person 1975) and Alicia Silva Pineda (e.g., Silva Pineda 1963, 1978) took on the study of Mexican fossil plants. However, it was not until the appointment of Reinhard Weber in 1972 at the Institute of Geology of the Universidad Nacional Autónoma de México (UNAM), that paleobotany was revitalized. Alicia Silva Pineda and Reinhard Weber produced important taxonomic work, focused mainly on Mesozoic plants, such as gymnosperms, ferns, and other groups of extinct plants that formed those communities (e.g., Silva Pineda 1970, Weber 1985; Figures. 1-6).
Time has made it clear that Mexico has been a special place for plants and that, trying to understand fossils from other areas, such as the USA or Europe, to explain the flora and vegetation of present-day Mexico, was not useful. One example that highlights this fact is Mexiglossa sp., a plant described based on its leaves by Delevoryas & Person (1975), whose taxonomic position remains a mystery. The difficulty of drawing direct comparisons with extant plants caused botanists to neglect Mexican fossils, instead of trying to understand current biodiversity through the prism of the fossil record and geology, accepting that Mexico has had characteristics in physiography, orography, hydrography, and geographical position that have resulted in communities that have differentiated from other floras of the world. Indeed, Mexico today has a great number of endemic plant species (Villaseñor 2016) and this diversity would be hard to understand without studying its fossil record.
The study of fossil plant communities from other latitudes has been extrapolated to understand Mexico’s current biodiversity, but this approach turned out to be insufficient for generating strong hypotheses on the origin of the extant vegetation of Mexico. Furthermore, comparisons between tropical, subtropical, or temperate forests, savannas, deserts, and xeric plant communities from Mexico and similar communities elsewhere support the distinctiveness of the vegetation of Mexico. This condition was not different in the past. For example, modern pine forests may look alike worldwide, but in detail, those from Mexico differ drastically from pine forests in Sumatra, the United States or Europe, when taxonomy and even ecology are considered (Farjon 2005).
Fossils are individual components of communities that developed and adapted to particular environmental settings at different points in time (Figures 1-6). Thus, they can help understand environmental changes responsible for community composition over time. Yet in Mexico, fossil plants have made a limited contribution to understanding modern biodiversity because only few researchers have studied them (Weber 1985). Nevertheless, this has been changing in recent years and their taxonomic value has been increasingly recognized in geological and biological studies of Mexico and the rest of North America, as they can help describe natural processes and settings (physiography, hydrology, climatology, etc.) that generate diverse plant communities (e.g., Pérez-García et al. 2012). Thus, fossil plants help understand current biodiversity through the joint discussion of paleobiology and geological processes.
Sources of information
Our review of the fossil record of plants from Mexico and around the world focuses on palaeobotanical work to understand the biology, evolution, and distribution of past plants to explain the presence of modern plants. We neither present a complete history of plant evolution nor an in-depth review of all Mexican fossil plants, but rather discuss examples to address the history of Mexican vegetation and to demonstrate how our biological concepts on biodiversity are often enriched when the fossil record is considered.
The application of paleobotany to other fields of paleobiology has made important progress over time, from the first descriptions based on general comparisons with current plants (Velenovský 1882, Fontaine 1889, Teixeira 1944) to the use of different statistical tools and phylogenetic methods to support the taxonomic identification and integration of fossils into phylogenetic systematics (Liang et al. 2016, Wang et al. 2017, Schönenberger et al. 2020, Friis et al. 2021, Wheeler et al. 2022). In addition, the re-evaluation of morphological information of fossils has prompted the creation and use of models capable of estimating the environmental conditions under which the plants grew, as well as describing physiological characteristics of the plants (Martínez-Cabrera & Cevallos-Ferriz 2008, Spicer 2009, Váchová & Kvaček 2009, Yang et al. 2011, Richey et al. 2021, Vornlocher et al. 2021).
The process of identifying macrofossils of isolated plant organs (Figures 1-6), that is, those that are dissociated from other organs of the whole plant, has commonly been carried out by comparing their characteristics with those of current and fossil taxa, those that the researcher considers to be related to the fossil. We recognize that microfossils, such as pollen and spores, are the most abundant component of the plant fossil record, but they have been identified generally with relatively low taxonomic resolution (Sauquet et al. 2012); for these reasons, in this work we focus mainly on macrofossils.
In recent years, interactive databases have been developed to facilitate the identification of isolated vegetative organs. Among the most famous is Inside Wood (insidewood.lib.ncsu.edu), which helps identify both current and fossil wood by comparing anatomical features of approximately 5,800 extant species and 1,600 fossils (Wheeler 2011).
For identifying isolated fossil leaves, the leaf architecture must be compared among many taxa, especially given the enormous morphological variation in both extant and fossil angiosperm leaves (Figures 3-5). The resources used for the identification and paleoecological interpretations of fossil plants by their leaves are varied and include: (1) herbarium specimens which can be accessed through digital media such as JSTOR (plants.jstor.org) and the Global Biodiversity Information Facility (www.gbif.org), or physically in different herbaria around the world (Merkhofer et al. 2015, Wilf et al. 2017, Jud et al. 2021, Xie et al. 2021); (2) other fossil publications (Greguš & Kvaček 2015, Golovneva 2018, Wang & Dilcher 2018); (3) cleared leaf specimens (https://www.geologia.unam.mx/igl/deptos/paleo/cevallos/html/hojas.HTML; Carvalho et al. 2011, Zhou et al. 2020, Zhu & Manchester 2020); and (4) databases that combine leaf information of extant and fossil plants (Traiser et al. 2018, Wilf et al. 2021). Identifying fossil plants through these methods has increased our knowledge of past diversity in Mexico. It is important to note that the evolution of this organ is currently not well understood, and possibly some characteristics of the leaf architecture could be due to evolutionary convergences related to environmental pressures common to different groups of angiosperms (Benton et al. 2021). Therefore, when many taxa are compared (both fossil and living), it is possible to observe and contrast the morphological variation between different groups, allowing for better identification of the fossils.
Mexican fossil wood and leaves have been identified based on anatomical comparisons with extant taxa (Magallón-Puebla & Cevallos-Ferriz 1994, Ramírez & Cevallos-Ferriz 2002, Calvillo‐Canadell & Cevallos‐Ferriz 2005, 2007, Estrada-Ruiz et al. 2010, Calvillo-Canadell et al. 2013, Velasco-de León et al. 2015, Centeno-González et al. 2019, 2021, Cevallos-Ferriz et al. 2021, Lozano-Carmona et al. 2021), and although this method is standard in Mexican studies, also statistical and phylogenetic tools are used to identify fossils and to model paleoclimatic parameters (e.g., Hernández-Damián et al. 2016, Rubalcava-Knoth & Cevallos-Ferriz 2021). These latter tools added certainty to the identifications and improved the morphological comparisons of multiple taxa, and thus allow the integration of fossils into classification schemes of modern plants (phylogenetic systematics), besides providing paleoclimatic and paleoecological data. Furthermore, statistical and grouping methods improve the establishment of taxonomic relationships and morphological variation between current and fossil taxa (e.g., cluster analysis, principal component analysis). These are supported by matrices of morphological data, with results represented through distance measures (e.g., Euclidean) and dendrogram-type graphs. These types of studies have been exemplified on leaves assigned to Sapindopsis sp. (Platanaceae) from the Sierra Madre Formation, Upper Cretaceous, Chiapas (Rubalcava Knoth 2019), and for the Oligocene leaves of Bauhcis sp. (Fabaceae), Cercocarpus sp. (Rosaceae), Karwinskia sp. (Rhamnaceae) and Pseudosmodingium sp. (Anacardiaceae) from the Pie de Vaca Formation, Puebla (Velasco de León et al. 1998, Ramírez et al. 2000, Velasco-de León & Cevallos-Ferriz 2000, Calvillo-Canadell & Cevallos-Ferriz 2002). We have also identified fossil wood using these methods, like the Miocene Tapirira sp. (Anacardiaceae) from El Cien Formation, Baja California Sur (Martínez-Cabrera & Cevallos-Ferriz 2004). An example of the application of phylogenetic systematics is the integration of the fossil record of Celastraceae (flowers, fruits, seeds, wood, and leaves) by Hernández-Damián et al. (2021; Figure 6), who further discussed their use in molecular clock calibrations. Similarly, Breña-Ochoa & Cevallos-Ferriz (2021) re-examined vegetative remains of Arecaceae plants using phylogenetic systematics to reconstruct complete plants (Figure 5). Although the phylogenetic systematics approach of the Mexican fossil floras is incipient, it represents a good starting point to reassess previous studies, as has been done in other parts of the world (Doyle & Endress 2010, Duan et al. 2020, Flores et al. 2021). Finally, ecological and paleoclimatic aspects of past Mexican vegetation have been supported by the Nearest Living Relative (NLR) method and the Structural Morphological Approach (SMA) (Martínez-Cabrera & Cevallos-Ferriz 2008, Martínez-Cabrera et al. 2014).
The Nearest Living Relative method depends on the taxonomic assignment of fossils, which is then correlated with the ecological requirements of their closest living relative species. This has been used to analyze plants embedded in Miocene amber from Simojovel de Allende, Chiapas, and estimate the paleoclimate of that area (Hernández-Hernández et al. 2020; Figure 6). Unlike the Nearest Relative method, the Structural Morphological Approach does not depend on taxonomic placement, because it uses morpho-anatomical characters of plants that are determined by ecological and environmental conditions, to build models that estimate paleoclimate or paleoecology (Wolfe 1993, Martínez-Cabrera & Cevallos-Ferriz 2008, Yang et al. 2011). Paleobotanists have focused on leaves and wood using this method.
Complex models such as CLAMP (Climate Leaf Analysis Multivariate Program; Wolfe 1993, Kovach & Spicer 1996, Spicer 2009, Spicer et al. 2011) relate leaf physiognomic characteristics of extant plants with the climatic conditions under which they live, making it possible to estimate paleoclimatic attributes of the paleofloras being analyzed (Kovach and Spicer 1996, Denk et al. 2022, Kafetzidou et al. 2022). CLAMP models have been applied to Cenozoic localities, like the Eocene La Popa (Carroza Formation), Nuevo León, or the Miocene Ixtapa Formation, Chiapas, and San Esteban (Tizatlán Formation), Tlaxcala (Hernández-Villalva et al. 2013, Domínguez de la Torre 2013). These estimations strongly suggest a change from the humid tropical conditions that prevailed during the Cretaceous, to the more seasonal and dry conditions that characterized the central and northern region of Mexico (seasonal dry tropics) throughout the Eocene and Miocene (Cevallos-Ferriz & Calvillo Canadell 2012).
Additional methods for paleoclimate estimations include linear regressions combined with anatomical characters that are intimately related to environmental functions, for instance, using leaves from the Upper Cretaceous Olmos Formation, Coahuila (Weber 1980, Estrada-Ruiz et al. 2008), and Oligocene and Miocene wood from El Cien Formation, Baja California Sur (Martínez-Cabrera & Cevallos-Ferriz 2008), and the Tehuacán Formation, Puebla (Rubalcava-Knoth & Cevallos-Ferriz 2021), respectively. Overall, leaf and wood physiognomy, along with paleoclimatic reconstructions, suggest that, in the past, Mexico hosted floras adapted to more humid conditions than today (Martínez-Cabrera et al. 2012, Martínez-Cabrera & Estrada-Ruiz 2014, 2021).
Although Mexican studies using numerical methods in plants are few, they help identify fossils more robustly and represent alternatives to better understand the history of the vegetation of Mexico, with ecological and climatic data complementing such history.
Aquatic primitive life
The fossil record of life on Earth undoubtedly begins with microfossils and biosedimentary structures (e.g., stromatolites, oncolites, sinters; Dunlop et al. 1978, Lowe 1980, Allwood et al. 2006, Djokic et al. 2017, Dodd et al. 2017; Figure 1). Since at least 3,800 Ma, microbes have colonized the planet and modified the biogeochemical cycles and ecology of the biosphere continuously until today (Knoll & Nowak 2017). The Mexican fossil record contains essential elements of this history, which document episodes of diversification that go along with unique environmental changes that have occurred for millions of years in what is now Mexico (Figure 1).
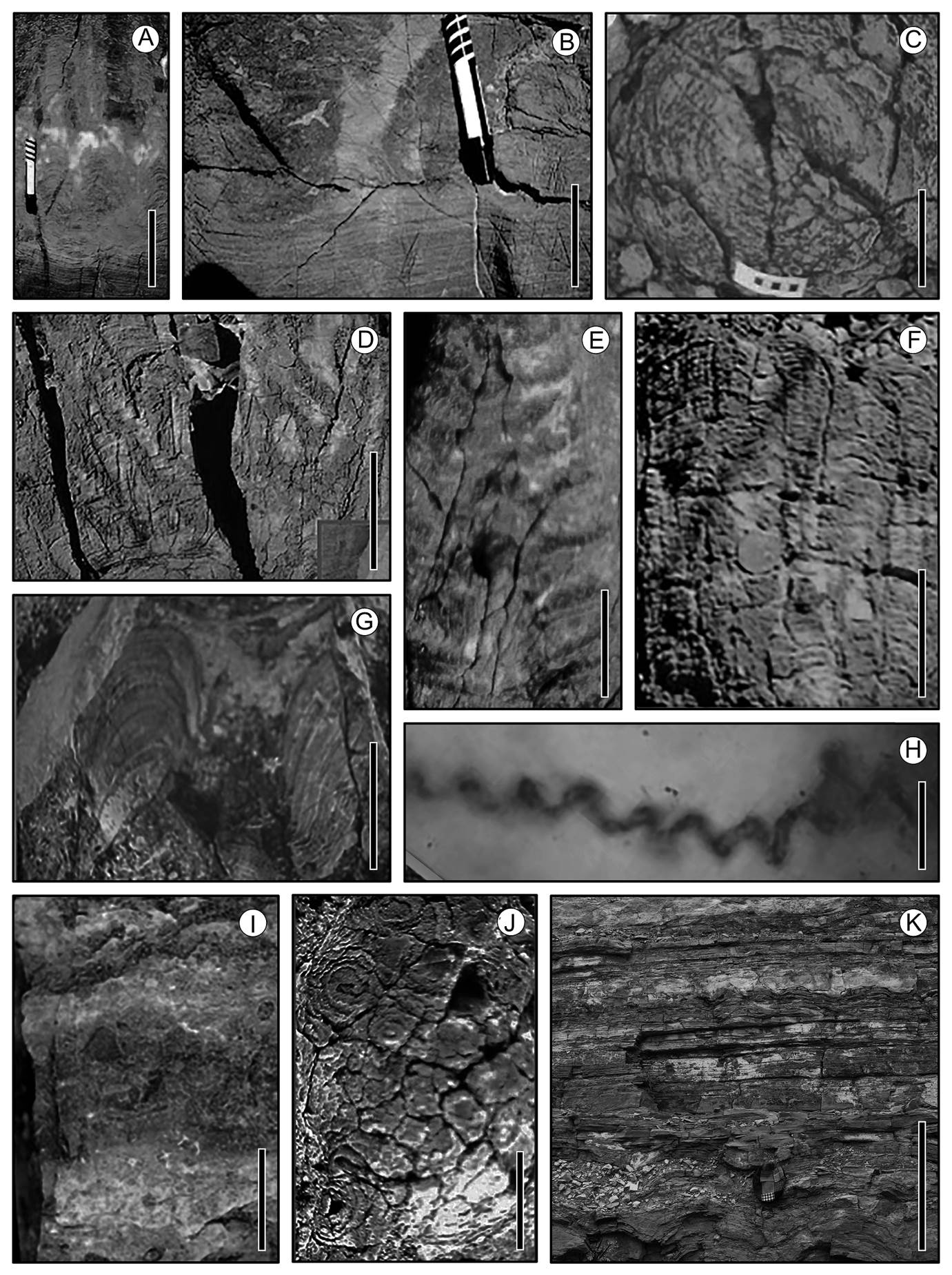
Figure 1 Organic sedimentary structures and a builder. (A)-(F) Precambrian, Caborca, Sonora; (G)-(I) Cretaceous, Huepac, Sonora; (J) Oligocene, Tepexi de Rodríguez, Puebla; (K) Cretaceous, Esqueda, Sonora. (A) Stratiform and columnar stromatolites in longitudinal section; scale = 13 cm. (B) Stratiform and irregular columnar structures; scale = 7 cm. (C) Conophyton cross section; scale = 8 cm. (D) Jacutophyton, column with branches in acute angle; scale = 25 cm. (E) Jacutophyton, column with branches at almost right angle; scale = 20 cm. (F) Table-like stromatolites; scale = 20 cm. (G) Domal stromatolite in longitudinal section; scale = 8 cm. (H) Cretaceous Spirulina-like organism; scale = 0.003 mm. (I) Laminated stromatolites with Chert intercalations; scale = 10 cm. (J) Stromatolites and mud-cracks around them; scale = 3 cm. (K) Sequence with alternating stratiform and domal stromatolites; scale = 90 cm.
The oldest sedimentary rocks in Mexico are found in the state of Sonora and are between 1,100 and 580 Ma in age (Anderson & Silver 1979, Stewart et al. 1984, Rodríguez-Castañeda 1994; Figure 1). They bear stromatolites (Weber et al. 1979) that have biostratigraphic importance to correlate similar structures in ~900 Ma-old rocks that crop out in Russia and North America (Cevallos-Ferriz & Werber 1980, Weber & Cevallos-Ferriz 1980; Figure 1), and that lived in shallow marine and platform environments that developed on the continental edges of the North American craton by the early Neoproterozoic. In the late 1970s, Reinhard Weber pushed forward studies on the oldest stromatolites of Mexico (Weber et al. 1979, Weber & Cevallos-Ferriz 1980). These were reported long before by Cooper & Arellano (1946), who described them from Caborca, Sonora, although they did not formally study them. Subsequently, Hugo Beraldi Campesi and Elizabeth Chacón Baca continued the study of the interactions between organisms and minerals in Mexican fossil assemblages (see the previous section; Figure 1). The stromatolitic reefs described by Weber & Cevallos-Ferriz (1980) are unique since the morphology of the ramifications of individual structures have not been reported from other locations in the world, except for similar structures in Russia (Krylov 1976, Serebryakov 1976) and Argentina (Poiré 1989). These morphologies are determined by the energy of currents, water chemistry, and the ability of microbes to trap particles (Dupraz et al. 2006, Jahnert & Collins 2012). However, the type of organisms in the stromatolites also seem to be a determining factor for their development (Serebryakov 1976, Jahnert & Collins 2012). In this sense, the Sonoran stromatolites call for further comparative studies of the type of sediments and macroscopic and microscopic morphologies that characterize them, to then formulate hypotheses about the type of environments that existed on the shores of that craton in the Proterozoic. Future studies should also search for the presence of microfossils, to explore their quality of preservation and their diversity (e.g., McMenamin et al. 1983), considering that photosynthetic eukaryotes were already present in the environment (Sforna et al. 2022).
Developing comparative studies would allow knowing and discussing unique biological phenomena in the past. For example, understanding how different reproductive strategies and life cycles were established for different organisms, which translate into diverse life forms and adaptations to particular environmental settings, some of which have been preserved (e.g., reproductive/vegetative structures or life cycles; Beraldi-Campesi et al. 2015). Through fossils, it is possible to infer how organisms were taking advantage of new ecological conditions that were not present earlier to generate new biodiversity and interactions with other organisms and the surrounding environment, for instance, organisms that evolved mechanisms to store silica inside their cells without using it (such as in algae and cyanobacteria, Riedel & Nelson 1985, Baines et al. 2005) and those that incorporated it for their ecological functioning (such as Radiolarians and Diatoms; Thamatrakoln & Hildebrand 2008). The same applies to the physiological changes that allowed organisms to transition from aquatic environments to land, using novel adaptative mechanisms, such as producing pigments capable of blocking harmful UVB and UVC light (Gao & Garcia-Pichel 2011), the modification of their exopolysaccharide (EPS) secretions for desiccation resistance and changes in salinity and pH (Decho 2000), or adaptations for living in clastic environments exposed to desiccation (Garcia-Pichel & Pringault 2001, Garcia-Pichel & Wojciechowski 2009).
Recent studies of the Cambrian of Sonora have revealed that the same coasts of the North American craton, where the stromatolites described by Weber et al. (1979) developed, underwent drastic changes in their configuration, currents, and energy, giving way to extensive coastal and tidal lagoons. The stromatolites here were replaced by thick horizons of oncolites, with a great variety of invertebrates and ichnofossils, together representing significant environmental changes in terms of physical conditions, spatial distribution, trophic chains, and primary productivity (Beraldi-Campesi et al. 2018). Yet again, more in-depth studies are needed to understand how the ecology of these communities worked and what impact they had on the surrounding biosphere, locally and regionally. This requires a better understanding of the lithologies and their fossil content, and comparative studies with coeval biotas from other regions of the world. With such studies, Mexican fossils could provide exciting and complementary information on the Ediacaran faunas and the Cambrian explosion, which generated the major animal phyla recognized today (Budd 2013), as well as others that became extinct, and even species that could have been endemic to the Mexican territory (e.g., Rajonia ornata, Devaere et al. 2021). Rocks of this age in Mexico have not been explored thoroughly and existing studies have addressed mostly the invertebrate faunas, but this rock record could contain traces of the first disarticulated vascular plants of the Lower Paleozoic, which would be crucial for discussions on their meaning and evolutionary history, given the geographic location that they would have had in the past.
Microbialites (e.g., stromatolites, oncolites, thrombolites; Figures 1) have an extensive record in Mexico and are essential because they provide valuable information about their environment. Yet, researchers have described fossil microbialites only briefly, treating them as lithological components of regional geological studies, without further detailed studies. For example, descriptions of stromatolites from the El Mármol locality in Baja California (~240 Ma; Buch 1984) do not include photographs, petrographic sections, or chemical analyses, which could have provided details on the marine environment represented by those rocks. Another example is the lack of microfossils in stromatolites from Tepexi de Rodríguez, Puebla, which appear in two separate strata, including small domes (10 to 15 cm high) in the lower stratum, and larger (up to 60 cm in height) club-shaped stromatolites in the upper one (Beraldi-Campesi et al. 2006; Figure 1). Their morphologies indicate that the former developed in shallow areas, while the latter did so in deeper waters of an Oligocene, saline lake, some 32 Ma ago in central Mexico (Beraldi-Campesi et al. 2006). Yet, their ‘biology’ was assumed based on the sedimentary structures per se, and not on the type of microbes that existed. In contrast, other studies focused more on the microfossils than on the sedimentary structures (Beraldi-Campesi & Cevallos-Ferriz 2005) because the type of environment favored the preservation of cellular structures (Figure 1H) and therefore, allowed better understanding of the kind of environment and ecology of the organisms than the microbialites themselves. In this case, more than 50 types of microfossils, permineralized in silica layers of hydrothermal origin (Rodríguez Ramírez 2011), were deposited in continental basins that were affected by volcanism by the end of the Cretaceous (González-León et al. 2011; Figure 1). Microbialites, such as stromatolites and oncolites, are also part of that record (Lucas et al. 1995, Beraldi-Campesi et al. 2004), as well as some of the oldest known non-marine diatoms (Beraldi-Campesi et al. 2015). The description of such microfossil assemblages resulted in the identification of algae, cyanobacteria and benthic and planktonic habits of the different morphospecies, which has enriched the value of this fossil locality. In addition, given their excellent preservation, it is likely that part of their organic matter was preserved, allowing for inferring particular metabolisms (see applications in Sforna et al. 2022) and, therefore, details of their biology that otherwise would remain speculative or unknown. Also, numerous plants from these ‘Huepac Chert’ rocks, such as palm trees and Haloragaceae plants (Cevallos-Ferriz & Ricalde-Moreno 1995, Hernández-Castillo & Cevallos-Ferriz 1999), with the same ultrastructural detail of preservation as the microfossils, have been described.
Primitive terrestrial life
Life on land masses must have begun in the Archean, more than 3,400 Ma ago, since there are records in Australia of sedimentary structures mediated by microorganisms, which developed in coastal areas that experienced desiccation (Noffke et al. 2013). This notion has been further supported by studies of more recent rocks (3,200 Ma) from South Africa, of sediments that accumulated in fluvial areas (Homann et al. 2015, 2018) and from these it has been possible to study behaviors of cyanobacteria that responded to phototropism, similar to modern cyanobacteria (Homann et al. 2015). In addition, the presence of paleosols found in rocks as old as 3,400 Ma (Beraldi-Campesi & Retallack 2016) suggests that the development of life on land had already begun on the Archean continents (Retallack & Noffke 2019). Although the land masses back then could have been relatively small compared to the size of the Earth (according to paleogeographic models of supercontinent assemblage (e.g., Hazen et al. 2015, Young 2013), for microbial colonies they could have represented enormous territories with countless possibilities in terms of moisture, energy, and materials for their reproduction and diversification. The biocrusts (Bowker et al. 2018), not yet described from the Mexican fossil record, are another source of information that could inform us about the formation of the first soils and microbial communities on land, where vascular plants later evolved. Thus, studying modern and fossil examples of soils and sedimentary structures developed by microbes (e.g., Beraldi-Campesi et al. 2015) is another area of opportunity in Mexico to broaden the understanding of past life and its impact on the biosphere.
Currently, several sources of evidence indicate that microbial diversity was abundant (in terms of mass, distribution, and metabolism) in shallow and deep marine environments at 3,500 Ma (Lowe 1980, Walter et al. 1980, Schopf 1983, Walsh & Lowe 1985, 1999, Westall et al. 2011, Tice & Lowe 2004, Allwood et al. 2006, Wacey et al. 2011). This evidence indirectly implies that metabolically diverse microorganisms could have also colonized continental, aquatic environments (lakes, rivers, swamps, etc.) and, from there, moved to terrestrial areas (Beraldi-Campesi 2013, Homann 2019). In this respect, Beraldi-Campesi (2013) suggested that terrestrial life evolved in parallel to aquatic life and not billions of years later. Mexican Precambrian rocks may show how crucial microbial biodiversity is (which awaits its documentation and exploration) for the many processes that must have governed their ecological interactions in the past.
Microorganisms have undoubtedly dominated biological evolution in terrestrial environments for more than 2,500 Ma (Beraldi-Campesi 2013, Beraldi-Campesi & Retallack 2016) and it seems that the Neoproterozoic was a pivotal period for drastic changes towards a more pronounced photosynthetic cover on the continents (Knauth & Kennedy 2009). However, some Proterozoic sedimentary basins show that the accumulation of organic matter in soils could also have occurred on the primitive Earth, more than 2,000 Ma ago. Such is the case of part of the Witwatersrand sequence in South Africa and Mount Roe in Australia, where the organic matter content of specific horizons is comparatively higher than that of the rocks deposited before and after (Rye & Holland 2000, Watanabe et al. 2000, Mossman et al. 2008). Although the record of paleosols is biased by the availability of the rocks where they were preserved, their increased frequency in younger rocks, as well as the increase in organic matter in them and their appearance in different types of Precambrian environments (Beraldi-Campesi & Retallack 2016) coincides with the formation of supercontinents at this time (Condie 2004). This latter process implies a significant increase in land areas and opportunities for microbial colonization of different terrestrial niches. These observations are essential because we can infer that such accumulation of organic matter in ancient soils required a long time and a lot of biomass, implying that microbial communities on land were not necessarily thin and dry biological crusts, but mature and abundant communities, sometimes far from water bodies, and perhaps more diverse than we think.
It is interesting to note that the morphology of some microfossils described from the Witwatersrand basin is very similar to fungal hyphae (Hallbauer & van Warmelo 1974), which are thought to have been very important for early land plant evolution (Pirozynski & Malloch 1975), and probably have been present on land much earlier (250 to 1,000 Ma) than plants themselves (Wang et al. 1999, Taylor & Berbee 2006). All this is important for Mexico’s geological and fossil record because its Precambrian rocks are as old as the transition towards that ‘prominent terrestrialization’ of the continents (Knauth & Kennedy 2009, Strother et al. 2011, Sheldon 2012). The sedimentary record of Mexico indeed contains essential elements for a better understanding of this history. For this reason, it is crucial to revisit many outcrops with new paleontological methods. An example of the potential in Mexico is the sandstones of the Las Víboras Group (Stewart & Poole 2002, Schopf 1983), Sonora, where according to Hugo Beraldi Campesi (personal observation), there are surfaces with traces of desiccation that relate with surfaces exposed to the atmosphere in fluvial environments from which fossil biocrusts could be identified. It is precisely in Sonora where most of the Precambrian rocks of Mexico are located, which are essential to understanding the basement’s tectonic and geological history between the Caborca and Mazatzal blocks (González-León et al. 2011). The study of the geologic evolution of Mexico could benefit from the fossil content of those rocks. We foresee great research opportunities for this area in terms of paleontological contributions to understand how the sea, the land, the inland water bodies, and life forms interacted for so many years, to eventually end up in what we see today as one of the most megadiverse territories on Earth.
Primitive plants
Fossil plants are generally thought as vascular plants (Figures 2-6), but curiously, paleobotanists often include algae, bryophytes, and fungi in their catalogs. In Mexico, only one researcher (Dr. Palmira Bruner) has extensively studied fossil algae, because at some point, algae were essential for oil exploration projects of the Mexican Petroleum Institute (e.g., Brunner 1975). Unfortunately, when she left her lab, this research area was cut short. Sometime later, Hugo Beraldi Campesi began studies on microorganisms and sedimentology that also included algae, but then broadened his scope into other microorganisms. Mexico lacks specialists on fossil algae, bryophytes and fungi, which sadly have been neglected, despite being present in the record (e.g., Magallon-Puebla & Cevallos-Ferriz 1993). On the other hand, studies of past life have not always been very focused on the organisms, but in their use as biostratigraphic indicators, supporting geological studies, which is not a very exact method for dating nor for generating paleoecological inferences based on few leaves or woods (e.g., Ramírez & Cevallos-Ferriz 2002). Climatic and environmental inferences need more elaborated approaches (e.g., Beraldi-Campesi et al. 2006, Martínez-Cabrera & Cevallos-Ferriz 2008, Breña-Ochoa & Cevallos-Ferriz 2021).
The study of the relationship between Geology and Biology has continued with fossils of more recent times. We can only know some aspects of the continuous innovation that generated unique and unrepeatable plant phenotypes and landscapes through the fossil record, which we will briefly describe. Understanding peculiarities of the biology of past plants clarifies that their functioning and associations have changed through time to generate their current biodiversity. Some key aspects of this history include the evolution of photosynthetic organisms (e.g., Sforna et al. 2022) and their ability to establish themselves on continents through anchoring and absorption systems (e.g., Lu et al. 2020), depending even less on water (Schoen et al. 2019), as well as developing conduction systems to move water from the ground to aerial parts.
Studies of macroscopic photosynthetic organisms from the Silurian-Devonian show that early land plants did not have roots, leaves, or vascular systems. In contrast, during the Carboniferous, groups like the Pteridosperms, Progymnosperms and Coniferophytes developed gymnospermic roots (e.g., Hetherington et al. 2016, Hetherington 2019). This difference suggests that plants had evolved new organs and improved their plant-soil interactions. Their relationship with the atmosphere also changed, as they developed megaphylls and wood, modifying their photosynthetic rate, and thus their CO2-O2 exchange rates. This likely had an impact on the biosphere, the lithosphere and the atmosphere (e.g., Doyle 2013, Kelly et al. 2016, Rothwell et al. 2014). Their reproductive structures also changed over time, achieving better protection for the gametophyte by the sporophyte to the point of retaining and protecting it, forming a seed, and even reaching the differentiation of the fruit.
The importance of roots
Most plants require roots to anchor and absorb water and minerals, which implies interactions between plant tissues and rocks and minerals. Roots are essential organs that early land plants had to develop to live on continents, on the way to the differentiation from non-vascular to vascular plants and were responsible for half of the work involved in colonization of the land at that time. Roots interact with the lithosphere to deliver water and nutrients to the aerial parts of the plant that interact with the atmosphere, where the other half of the innovations occurred (e.g., Raven & Edwards 2001, Shekhar et al. 2019). For a long time, it was thought that the first algae-plants that came out of the water and were exposed to the atmosphere did not have roots but used simple branching axes that grew from the epidermal cells, as rhizoids. However, this idea has changed in the last decades, and an interesting hypothesis has been proposed (e.g., Hetherington & Dolan 2018), which suggests that the first roots corresponded to horizontal branching axes with positive geotropism. This is based on anatomical sections of fossils, that have areas with cells that are different from those of the cortex and epidermis, corresponding with sites where rhizoids develop. These are interpreted as lateral meristems that produced rootlets with positive geotropism, suggesting that such parts behaved like modern roots but without departing from a sub-apical meristem and lacking a root cap (e.g., Kelly et al. 2016, Matsunaga & Tomescu 2016). This morphoanatomical arrangement of the axis could represent an early stage in the evolution of the root.
The few Mexican outcrops with Devonian plants known from Cañón de la Peregrina, Tamaulipas, have not been investigated in detail yet. However, a group of paleontologists from the Museum of Paleontology of the Faculty of Sciences, UNAM, a couple of decades ago collected axes that looked like parts of lycopods, but this material was insufficient for a thorough study. Indeed, fossils from Cañón de la Peregrina could contribute to this critical discussion and concepts alike of the early vascular plants. Studies of this kind seem also important for current climate issues and sustainability, as roots were and are an essential element to contain soil erosion; they are important modifiers of the lithosphere that generate spaces where microorganisms develop and capture CO2, avoiding greenhouse effects, and capture moisture, modifying the lithosphere-atmosphere interface (e.g., Kenrick & Strullu-Derrien 2014). Fungal hyphal networks also use plant root systems to transport organic and inorganic materials to distant areas, expanding soil biomass by orders of magnitude and having implications for bioremediation (Banitz et al. 2013). Roots are soil-forming agents, and soil is our main source of food and home to a remarkable biodiversity (a quarter of it). It represents a non-renewable resource that takes hundreds or thousands of years to form, and only a year (or less) to erode. The importance of roots as an innovation for plant life is highlighted by the biodiversity hosted in soils that is responsible for many biological processes that sustain the life of many other organisms, including humans (e.g., Augstein & Carlsbecker 2018).
Fossil soils (paleosols) were found by Reinhard Weber in the Matzitzi Formation (Permian-Triassic) of Puebla, and in the Santa Clara Formation (Triassic) and the Olmos Formation (Cretaceous) of Coahuila (e.g., Weber 1972, 1980, 1985, 1999). Other sites with paleosols include various central and southern Mexico localities aged 30 Ma or less. Plant roots and their underground environment in the fossil record still have a lot of stories to tell, highlighting that the past and the present are closely related, but their differences remain a mystery. It is worth mentioning that the study of paleosols in Mexico has focused on those of archaeological interest, but ancient paleosols have yet to receive attention from an evolutionary standpoint.
Vascular tissues
The axes of early land plants were green, and pores and stomata, responsible for the exchange of gases and keeping their body temperature low, have been found on their surfaces. These two aspects are important because, in Silurian-Devonian time, the amount of CO2 in the atmosphere was relatively high, producing greenhouse effects with higher temperatures, which could have caused organisms to overheat (e.g., Brugger et al. 2019). These early plants had to build pipelines from their rooting systems to the apex of their axes during the Silurian-Devonian (e.g., Friedman & Cook 2000). Such pipelines then differentiated from living cells into structural, dead cells. Fossils demonstrate that the axes of the earliest vascular plants had a very well differentiated central zone, equivalent to the zone of the primary vascular tissue of more advanced plants. However, detailed observations have shown that what appears to be thickened secondary walls in these conductive cells, either lacked or had very little lignin (e.g., Boyce et al. 2003, Weng & Chapple 2010). Lignin deposits in tracheary elements of modern plants occur only in dead cells or cells in the process of dying. These deposits significantly increased the upward strength of early plants, which grew in height, and facilitated the transfer of water through the process of tension-cohesion-evaporation-transpiration. Those primitive conductive cells would later evolve into modern tracheary elements and, although both have similar functions, the physiology for the good performance of primitive pipes would have been different among the varieties of early plants, from which only some dominated until today (Edwards et al. 2006, Strullu-Derrien et al. 2013, Tomescu 2021).
Again, it is here where Mexican outcrops like the one in Tamaulipas may contain exciting information about these problems. Furthermore, it is necessary to walk and explore the rock sequences with Silurian-Devonian sediments in Sonora that could have important information about plants or their parts, expanding our idea about the colonization of the emerged areas of the continents. It would be essential to look for new outcrops where permineralization has favored the preservation of the internal structures of those fossil plants (e.g., Rodríguez-de la Rosa & Cevallos-Ferriz 1994, Hernández-Castillo & Cevallos-Ferriz 1999, Huerta Vergara & Cevallos-Ferriz 2018).
Increasing photosynthetic surfaces
During the Silurian-Devonian, the high concentration of atmospheric CO2 created a moment of opportunity for plant axes to expand their photosynthetic surfaces (e.g., Tomescu 2009). This process added functions for CO2 concentration mechanisms and the improved use of oxygen, consequently maintaining a low body temperature and improving pumping functions to raise and distribute water throughout the whole plant (Brugger et al. 2019). The photosynthetic ancestors of plants did not have these problems when they lived in the water. As organisms advanced toward dry lands, their biological processes had to adapt to new physical and chemical conditions. Projections of the cortex of the axes that first became enations and later leaves, worked to buffer the aggressive atmosphere (e.g., Tomescu 2009). There are two main types of leaves: microphylls (characteristic of Lycophyta) and megaphylls (all the other vascular plants). Of course, there are exceptions and several types of leaves within these two categories (e.g., Tomescu 2009).
Outcrops with leaves in pre-Permian times are not known in Mexico, but several examples of fossil leaves exist after that period (e.g., Velasco-de León & Cevallos-Ferriz 2000, Ramírez & Cevallos-Ferriz 2002, Hernández-Villalva et al. 2013; Figs. 2-5), and they represent an ample variety of plants, many of which have become extinct while others have persisted to the present. Though leaves by themselves do not define the size of the plants they came from, their identification and association with other organs can be used as proxies, which suggests that lycophytes and arthrophytes (currently represented by small plants) started as small herbs in the Devonian and grew to be large trees by the Upper Paleozoic. The ferns were herbaceous by the Devonian, reached shrub size by the Upper Paleozoic, and aside from the tree ferns, are small today. Gymnosperms started herbaceous and shrubby by the Upper Paleozoic and became large trees since the Mesozoic. This shows that plants followed different strategies in growing leaves and other organs and modifying their physiology to take advantage of the exposure to sunlight of their particular environmental settings.
The leaf fossil record of Mexico is represented by different vegetation types since the Permian, some of which are related to current forms. In this regard, studies by Weber, Silva Pineda, and Hernández-Castillo suggest that forests changed through the Mesozoic significantly (e.g., Rubalcava Knoth 2019), adopting different forms and colonizing habitats that differed in elevation and humidity. For example, Walchia sp. represents an important coniferous lineage by the Permo-Triassic, becoming even more frequent since the Jurassic (Weber & Zamudio-Varela 1995). The Bennettitales were the most diverse and dominant vegetation in the Jurassic but decreased in importance towards the Cretaceous. The Permo-Triassic and Jurassic arthrophytes and lycophytes were apparently minor elements of the vegetation, and the older ones appear to be more robust. Ferns of the Permo-Triassic were more diverse than Jurassic ones, but they have been scarcely studied (e.g., Velasco-de-León et al. 2020).
The work of Alicia Silva Pineda and Patricia Velasco-de León exemplifies the importance of knowing the Paleozoic and Mesozoic communities through plant leaves. Recently, Velasco-de-León et al. (2020) and Kelly et al. (2016) demonstrated the dominance of Bennettitales in floras of southern Mexico and recognized six different distribution patterns that changed both geographically and temporally. Jerónimo Morales Toledo added yet another perception on a flora of southern Puebla, in which conifers become an essential element, in contrast to the dominance of Bennettitales in the southeast of Oaxaca (e.g., Lozano-Carmona & Velasco-de León 2021, Morales Toledo 2019, Figure 2). Of course, they share other elements that suggest the presence of homogeneous vegetation throughout Mexico ~160 Ma, regardless of the geological processes that selected and differentiated them simultaneously. A similar situation occurs with current coniferous forests; although conifers dominate, components of communities from different sites make them unique and different, hence the importance of their characterization.
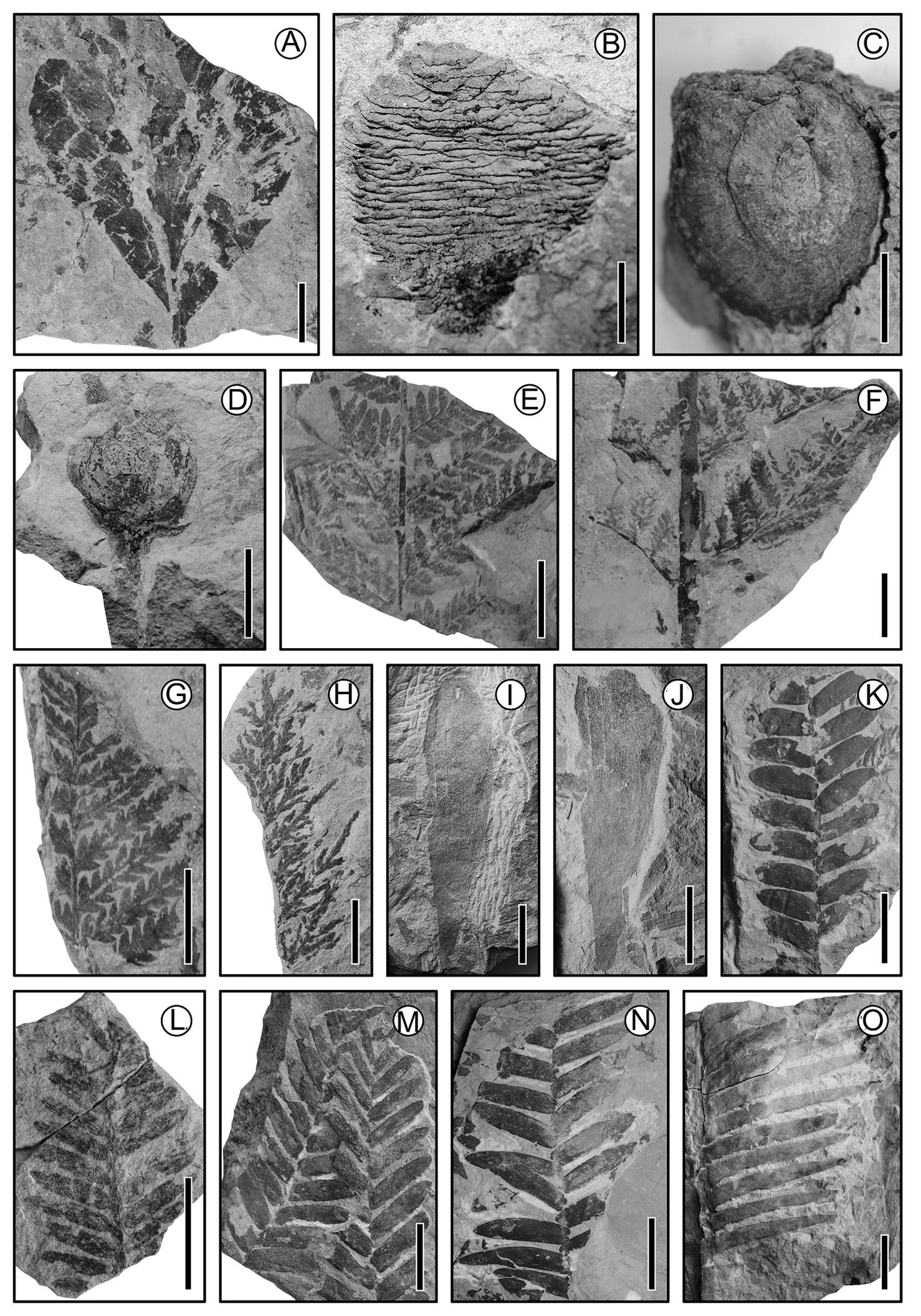
Figure 2 Jurassic material from San Esteban Tizatlán, Puebla. (A) Gingko leaf; scale = 2 cm. (B) Ovulate cone; scale = 1 cm. (C) cf. Williamsonia gigas (Bennettitales); scale = 2 cm. (D) Williamsoia (Bennettitales); scale = 2 cm. (E) cf. Aspidites (Pteridophyta); scale = 1 cm. (F) Fern frond (Pteridophyta); scale = 2 cm. (G) Fern frond (Pteridophyta); scale = 1 cm. (H) Fern frond (Pteridophyta); scale = 1 cm. (I) Heidyphyllum; scale = 3 cm. (J) Heidyphyllum; scale = 3 cm. (K) cf. Zamites (Bennettitales) leaf; scale = 2 cm. (L) Conifer? leaf; scale = 2 cm. (M) cf. Zamites (Bennettitales) leaf; scale = 2 cm. (N) cf. Zamites (Bennettitales) leaf: scale = 3 cm. (O) cf. Zamites (Bennettitales) leaf; scale = 5 cm.
Leaves are essential beyond their photosynthetic function. We have associated them with other organs documenting the evolution of reproductive structures. In some groups, the leaves protected the megasporangia, generating analogous structures similar to today’s seeds, such as in lycophytes and ferns (e.g., Rothwell & Erwin 1987, Meade et al. 2021). In arthrophytes, their compound cones, sometimes formed by leaves and branches, mimicked seed functions, but it is the branches, the sporangiophores, that protected the megasporangia. The telomes followed a third pathway; they fused themselves around megasporangia to form the integument in the seed plant lineage.
In the Mesozoic, there is evidence that pteridosperm leaves (seed ferns) tended to protect ovules, as described for Caytoniales, Glossopteridales, Peltaspermales, etc. (e.g., Westoby & Rice 1982, Taylor et al. 2006, Sauquet et al. 2017, Rudall 2018), not megasporangia. In Mexico, we have carried out a few studies that emphasize this. Still, seeds from the Permo-Triassic Matzitzi Formation, Puebla, require detailed investigations, and cupules compared to Caytonia sp. from the Jurassic of Oaxaca and Puebla deserve more attention, as they represent leaves that enclose the ovules.
Mesozoic biology of the groups mentioned above was surprisingly different from the Paleozoic one. Highlights include a more common occurrence of plant groups with bipolar embryos, dominance of determinate growth, diversification of bifacial vascular cambia and root types.
Although we have not mentioned reproductive strategies, it can be generalized that in the Silurian-Devonian, the homosporous plants dominated. In the Carboniferous-Permian, the heterosporous plants were relevant and diverse, and the seed habit developed and diversified, but in the Mesozoic plants with bare seeds (gymnosperms) dominated the vegetation, while other heterosporous plants, as well as homosporous plants, were essential elements but less abundant. (Vajda et al. 2016 and papers in the same issue of that journal). From Mexico, we could contribute to the understanding of all these processes and differences that make biology more exciting and dynamic over time.
Reproductive structures
Reproductive structures also changed through time, as the plant vegetative body did, achieving adaptations to the changing environment and allowing less dependence on water (e.g., Gerrienne et al. 2004, Matsunaga & Tomescu 2017, Bonacorsi et al. 2020, Renzaglia et al. 2000). The first land plants used spores of the same size, shape, and apparently physiology to reproduce. These characteristics suggest that the gametophyte that emerged from the spore grew outside the spore (exosporic) and produced both archegonia and antheridia. These processes could have either taken place at different moments on the same gametophyte or one gametophyte could have produced mostly male and another mostly female gametangia. These events may have happened at the same or different times. Furthermore, the same gametophyte may have produced antheridia first and archegonia later, to minimize self-fertilization. There are not many outcrops with Silurian-Devonian plants in Mexico, although few bear potential for documenting the reproductive biology of early land plants and vascular plants in the tropical areas of the world (e.g., Leslie & Bonacorsi 2022).
By the Carboniferous, homosporous plants gave rise to heterosporous plants that produced microspores with antherozoids (sperm cells), which had to be released in the presence of water to swim towards another gametophyte with archegonia (containing the eggs), to enter through its neck and fuse with the egg cell. In this case, the gametophytes of heterosporous plants were endosporic. Gametophytes were retained within the spores, exposing themselves to the environment as ecological conditions were suitable for swimming and moving to the archegonium or receiving the antheridium and carrying out fertilization (e.g., Petersen & Burd 2018, Wang & Bai 2019). Although heterosporous fossil plants from the Devonian have not been found in Mexico, plants from the Permian onwards could provide a better understanding on reproductive strategies and the resulting dynamics of past communities that grew in what is today Mexico.
Some of the heterosporous lineages reduced the number of megaspores per megasporangium to a single functional one, and three abortive ones. Later, a vegetative body structure was associated with this megasporangium and enveloped it, generating a protective layer. This protection of the megasporangium is the integument of seed plants, and the whole structure (megasporangium plus integument), corresponds to an ovule, from a morpho-anatomical point of view. Many types of studies have documented the presence of ovule-like structures and ovules in the fossil record in practically all plant groups (e.g., Renzaglia et al. 2000, Gerrienne et al. 2004, Rudall 2018, 2021). Still, the integuments do not have the same origin in all lineages. In the lycophytes, it is a microphyll that serves as protection, while in the rest of the lineages, it is megaphylls or branches that intervene in the formation of the ovule. So far, we must say that megasporangia with two, not one, as would be the typical case of ovules, functional megaspores are known in ferns (e.g., Stauropteridales). However, there are some taxa among angiosperms with two embryonic sacs which justifies the comparison.
On the other hand, though arthrophytes do not have ovules, branches that surround megasporangia are the sporangiophores, while in the lineage of seed plants, telomes are involved in the protection of megasporangia, forming true ovules (e.g., Herr 1995, Meade et al. 2021). Thus, the evolution of the ovule from a megasporangium as an extreme case of heterospory represents a process of great importance in the definition of the spermatophyte lineage (e.g., Rudall 2021). In Mexico, since the Permian, we have had plants represented by leaves and branches with associated reproductive structures (e.g., Silva Pineda 1984, Weber 1995, Ramírez & Cevallos-Ferriz 2002, Velasco-de León et al. 2019). These records open the possibility of participating in studies that have proven to be very important to understand and justify the decreased dependence of plants on water and facilitate their colonization of intra-continental areas.
Mexican plant fossils: a hidden treasure of knowledge on plant evolution waiting to be unearthed
The sum of adaptations in plants generated different communities over time. Comparing past plants with current ones and their floristic association improves our understanding of the changes that the communities underwent, leading to the present plant diversity (e.g., Iglesias et al. 2011, Gibling & Davies 2012, Contreras 2018). Along this history, there are fields of opportunity where Mexican fossils can contribute. For example, the few outcrops of Silurian-Devonian age can add important information about early plants, with rhizomatous axes and lateral meristems that served as roots (Weber & Cevallos-Ferriz 1994, Cevallos-Ferriz et al. 2012, Villanueva-Amadoz et al. 2014). Regarding developmental biology, there were plants with unipolar embryos, indicating that all plant growth came from a single pole of growth. In addition, their conducting tissues were different from those of extant plants and provided relatively little support for sporophytes, which did not produce leaves. The plants were small, and at this time, the Earth seemed to support communities that resembled “savannas or grasslands” in appearance but were morphologically distinct and functioned very differently. All this new life on the exposed continents was water-dependent and could not become established far away from water bodies. We have ventured little in Mexico to understand the communities that thrived in what is now Sonora and Tamaulipas, where we have pointed out the possibility of contributing to this area of research (e.g., Weber 1985, Zambrano García & Weber 1985). Still, the door is open to get to know how life established and grew 420-395 Ma ago in these areas.
As the Devonian advanced, the communities that grew on land masses consisted of plants that increased in size, and albeit still small, they were already approaching a meter in height; they had microphylls or megaphylls, some of them evolved heterospory, and a few had megasporangia with one functional megaspore as precursors of the current seeds. Though these plants scarcely had an integument surrounding the megasporangium, this second episode in the development of the continental plant communities brought them closer to the present ones. These communities from 395 to 360 Ma ago can be known in Mexico if we visit localities in Sonora and Tamaulipas where the reconstruction of the vegetation will undoubtedly surprise us and expand the ideas we have based only on current plants (e.g., Weber 1972, Weber & Cevallos-Ferriz 1980, Velasco-de León et al. 2019).
What we once believed was Carboniferous strata in Mexico now appears to be Permian or even Lower Triassic. Still, if we examine closer the communities described from these sediments, surprises will continue to emerge. Little by little, the lycophytes and arthrophytes were decreasing in importance within the communities, and herbaceous and shrub-like ferns became important. In the Carboniferous-Permian, the Pteridosperms (seed ferns), and the lineage that led to the conifers diversified. However, they are different, as shown by their cones, types of wood, and leaves. Towards the end of the Paleozoic a vegetation type well differentiated from the first terrestrial communities but still very different from the current ones was in place. In Sonora and Puebla, relevant outcrops invite us to study life about 260 to 240 Ma ago (e.g., Centeno-García et al. 2009). Although we have begun their study, we are far from having complete data on these communities.
It seems that from the Carboniferous onwards, we can notice a remarkable similarity between current and fossil communities. Unlike the Devonian, where homosporous plants dominated, the Carboniferous had heterosporous plants, among which seed plant diversity was significant. In the Carboniferous, root, leaf, embryo, and wood types diversified. The arboreal part of the communities reached up to 30 m in height or a little more. There are also shrubs, so in the Carboniferous vegetation three vegetation strata can be distinguish. We can also highlight that the typical gymnosperm roots were differentiated. Seeds were becoming more and more common, and therefore communities were able to move away more easily from water bodies, entering the interior of the continents.
The Mesozoic in Mexico is open to new adventures in terms of recognition of new taxa and vegetation types through time (e.g., Weber et al. 1980a, b, Weber 1985, Zambrano García & Weber 1985, Velasco de León et al. 2013, Hernández-Castillo & Cevallos-Ferriz 1999). Of course, the Triassic of Sonora is surprising due to the importance of lycophytes and arthrophytes, abundance of ferns, but above all because of the diversity of conifers already present. With the observations made so far, it is worth calling attention to Weber’s suggestion that this community is close to the Triassic communities of the Atlantic coast of the United States, rather than to the Triassic plants of Arizona, which according to their proximity, we would expect to have similar communities. There is still much to learn, but we can anticipate that extinct plants with cones and woods dominated these communities and resemble current conifer forests, but detailed observations confirm that they are different. In these communities, the presence of groups collectively referred to as Mesozoic Pteridosperms is notable because paleobotanists have discussed them as groups that could be sister to or part of the stem group of angiosperms. However, we do not know many details about these plants from the Mesozoic of Mexico due to the limited studies carried out on them to date.
Interesting Jurassic plants of central and southern Mexico are more numerous. Lycophytes and arthrophytes continue participating within communities, but their size is smaller, and conifers and ferns diversify (e.g., Morales Toledo 2019, Velasco-de León et al. 2019; Figure 2). The Jurassic communities represent a transition between those that arise from the Paleozoic and are present at the beginning of the Mesozoic and those differentiated from the Cretaceous at the end of the Mesozoic (e.g., Weber 1985; Figures 3-5). Communities through the Paleozoic and Mesozoic contained similar lineages. Still, the taxonomic participation, the size, and abundance of each of the members of these lineages in the different communities vary, and the vegetation types that developed over time also differs.
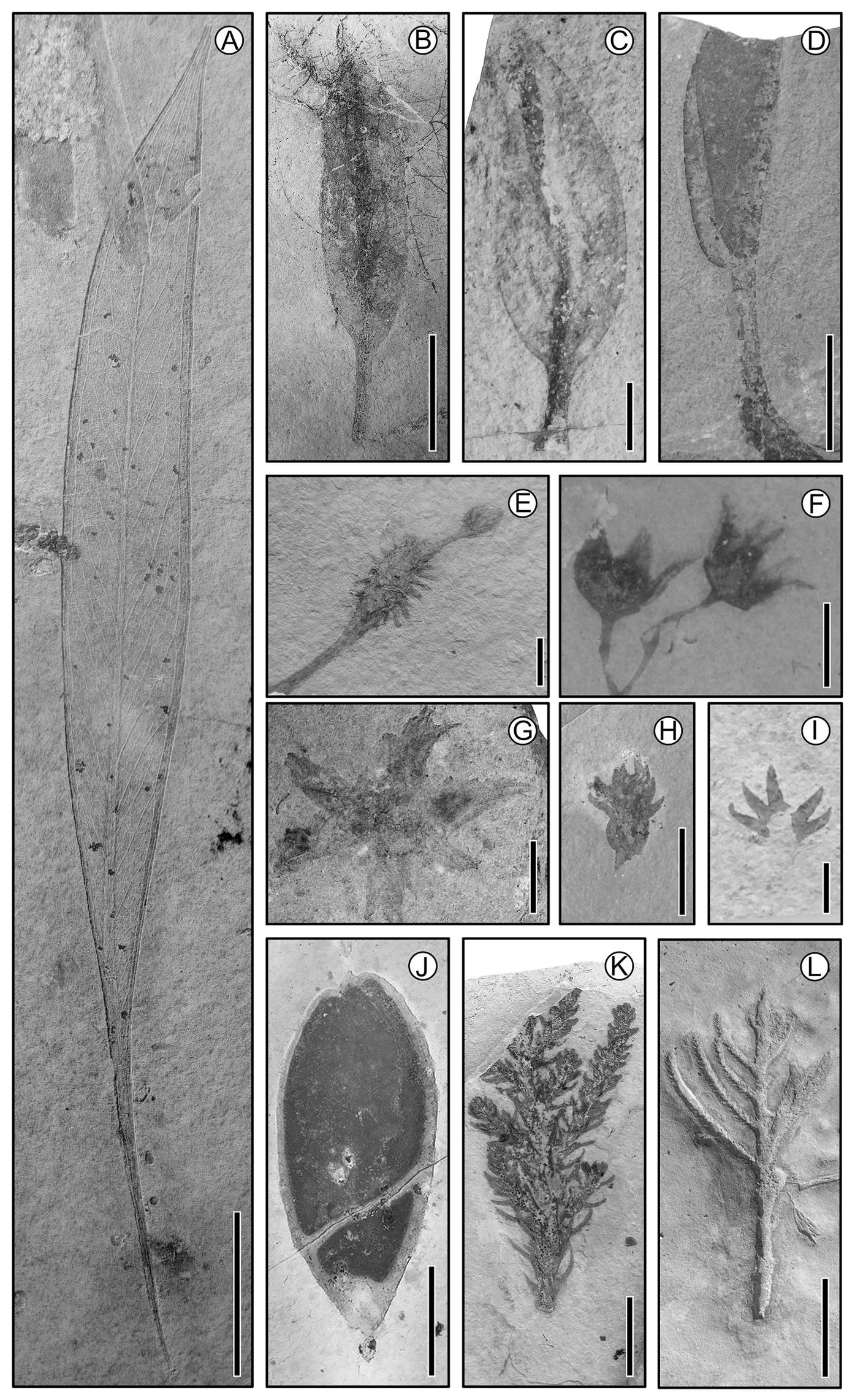
Figure 3 Fossils from the Sierra Madre Formation, Cretaceous, ca. 100 Ma, Chiapas. (A) Platanaceous (Sapindopsis?) leaf; scale = 2 cm. (B) Leaf of unknown affinity; scale = 2 cm. (C) Bract from which a flower (not in photo) hangs; scale = 1 cm. (D) Fruit with dehiscent line; scale = 2 cm. (E) Fruit of unknown affinity; scale = 1 cm. (F) Flowers with nice perianth; scale = 1 cm. (G) Bracts sustaining a fruit; scale = 0.5 cm. (H) Conifer cone; scale = 2 cm. (I) Bract of a conifer; scale = 0.5 cm. (J) Fruit; scale = 2 cm. (K) Conifer branch with long leaves; scale = 1 cm. (L) Conifer branch with short leaves; scale = 1 cm.
The distinct Mesozoic groups continue to differentiate generating specific communities, but some became extinct - the Pteridosperms (e.g., Glossopteridales, Caytoniales, Peltaspermales, Pentoxylales, etc.). In addition, many conifers evolved and became extinct at different times in the Mesozoic. Under this perspective, it is possible to imagine similarities and differences among the communities and plants over time, becoming more like the current ones as we get closer to recent times. Nevertheless, it is necessary to value the vast taxonomic, morphological, anatomical, and association differences that distinguish them, all of which become challenges as exemplified by the discussion on the origin of angiosperms above (e.g., Scutt 2018, Li et al. 2019).
In the Cretaceous, communities continued to change. They had plants and vegetation even more like current ones due to the differentiation of angiosperms that dominate practically all ecosystems. Therefore, the importance of many of the groups that preceded their appearance decreased. However, we should mention that although the distribution of gymnosperms was restricted, their presence was significant. The existing distribution of the group in Mexico, e.g., conifers mainly in upper parts of the mountains and cycads in mesic and dryer areas, are not the best examples to describe their past communities and ecosystems. It is necessary to bear in mind that in Mexico lower Cretaceous plants became extinct. At that time, the established communities and lineages of gymnosperms and flowering plants, among others, have relevant differences compared to recent ones (Figures 4-5). Plant representatives of the Paleocene may correspond to current genera, but the species are clearly different, and the vegetation is also distinctly different from the extant one. The extant species are of relatively recent appearance. In the case of Mexico, paleobotanists have suggested that both the present vegetation and recent species did not establish in the region earlier than 5 Ma ago (e.g., Huerta Vergara & Cevallos-Ferriz 2018, Hernández-Damián et al. 2021). Before this moment, the communities resemble current ones, and the species may be close, but neither are the same (Figure 6). The corollary of this condition is the proposals of the Arcto-Madro Tertiary or the Boreotropical flora concepts that explain the particular vegetation and floras that dominated the northern hemisphere during the Cenozoic (e.g., Cevallos-Ferriz et al. 2012, Huerta Vergara & Cevallos-Ferriz 2018).
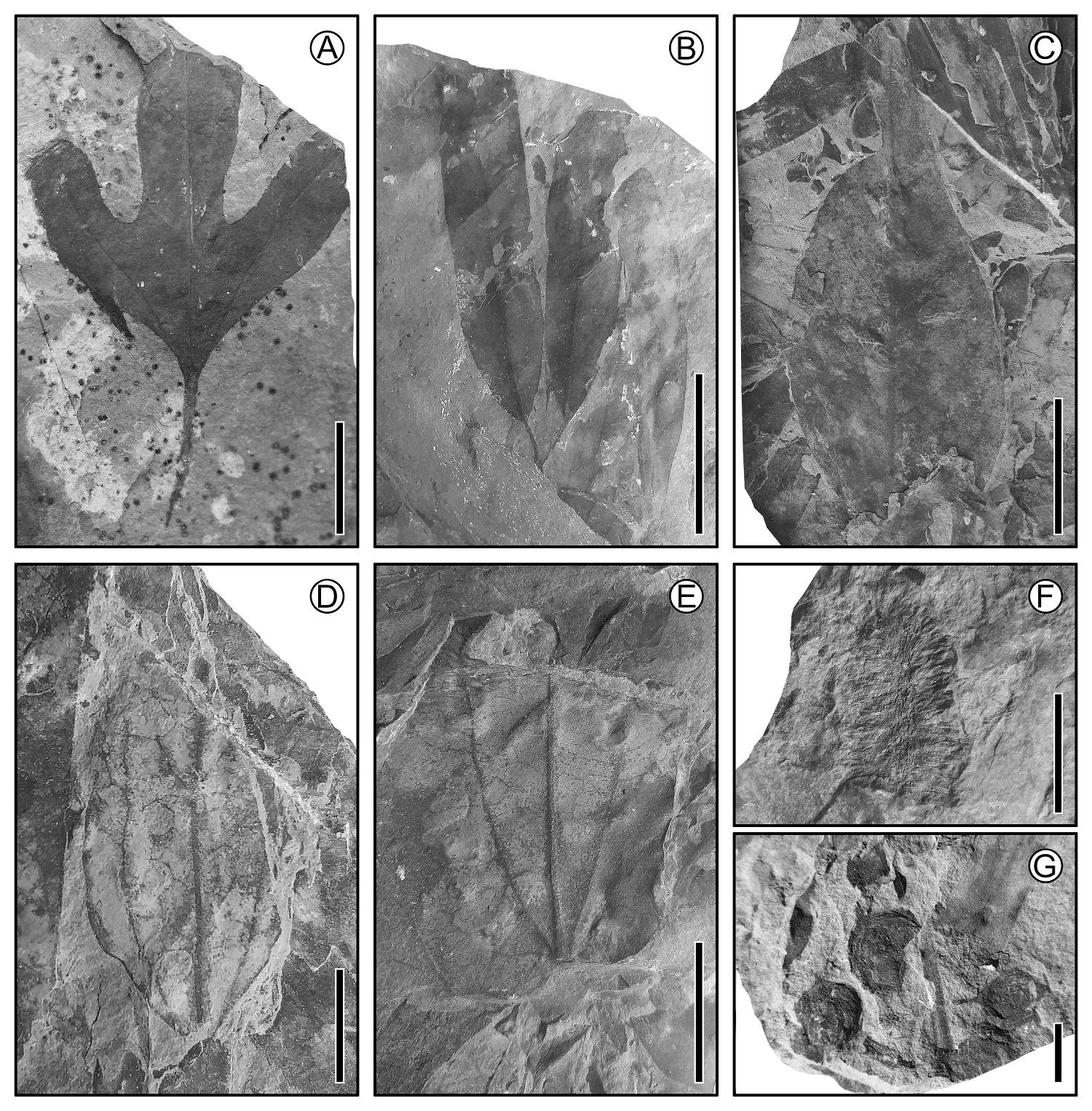
Figure 4 Cretaceous fossils plants from Cabullona, Sonora. (A) Lauralean leaf (Araliaephyllum); scale = 2 cm. (B) Leaves attached to a twig; scale = 2 cm. (C) Leaf with pronounced acuminate apex; scale = 2 cm. (D) Leaf with acrodromous primary vein framework; scale = 2 cm. (E) Lauraceous leaf with three principal veins; scale = 2 cm. (F) Inflorescence; scale = 1 cm. (G) Fruits; scale = 1 cm.
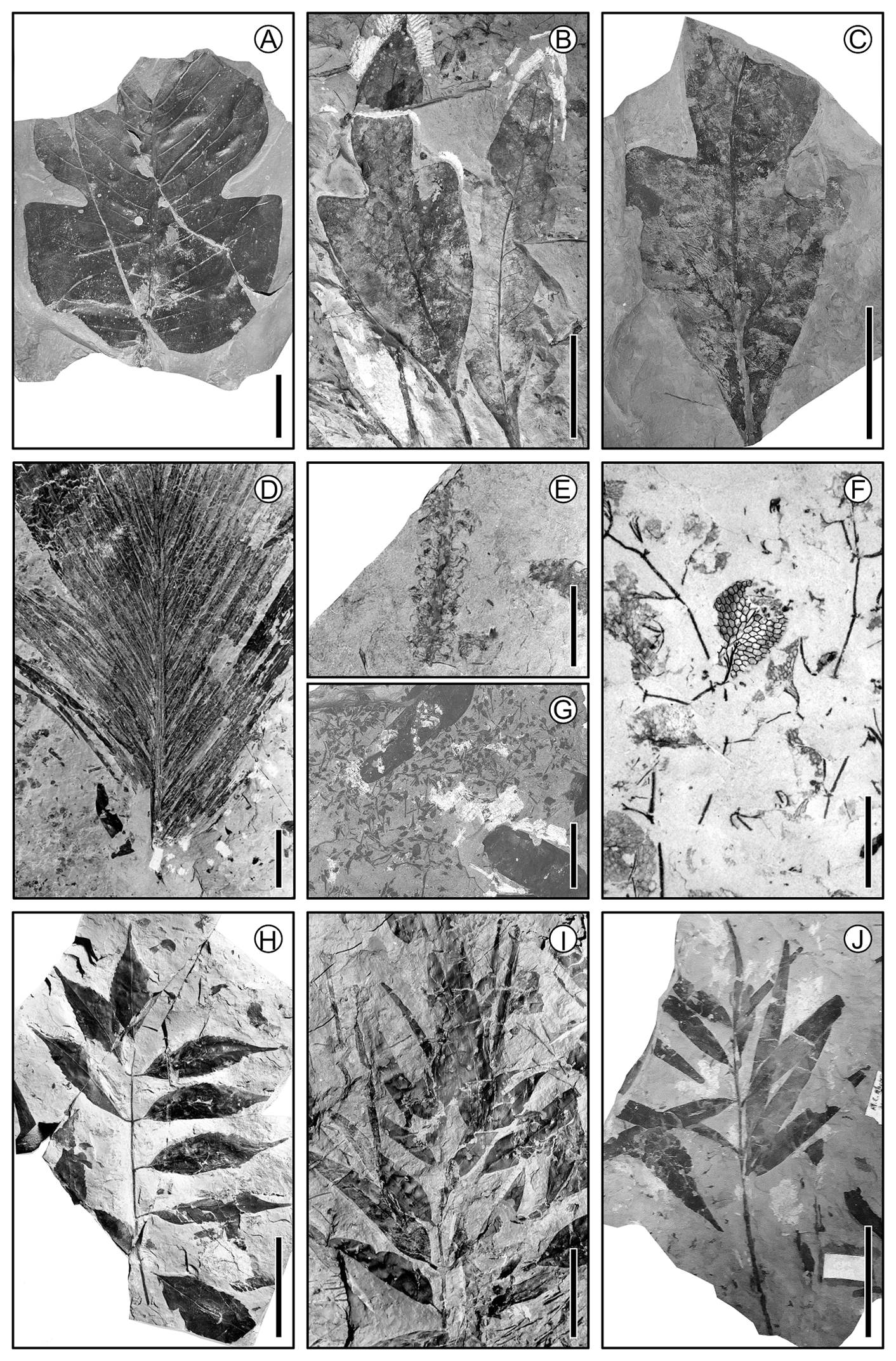
Figure 5 Fossil plant material from the Cretaceous carboniferous zone of Coahuila, 70-65 Ma. (A) Magnolian bilobed leaf; scale = 2 cm. (B) Lauraceous leaf; scale = 4 cm. (C) Lauraceous leaf; scale = 4 cm. (D) Palm leaf; scale = 10 cm. (E) Catkin; scale = 1 cm. (F) Aquatic fern; scale = 0.5 cm. (G) Many small flowers; scale = 2.5 cm. (H) Compound leaf with serrate margin leaflets; scale = 5 cm. (I) Twig with leaves; scale = 5 cm. (J) Twig with leaves; scale = 5 cm.
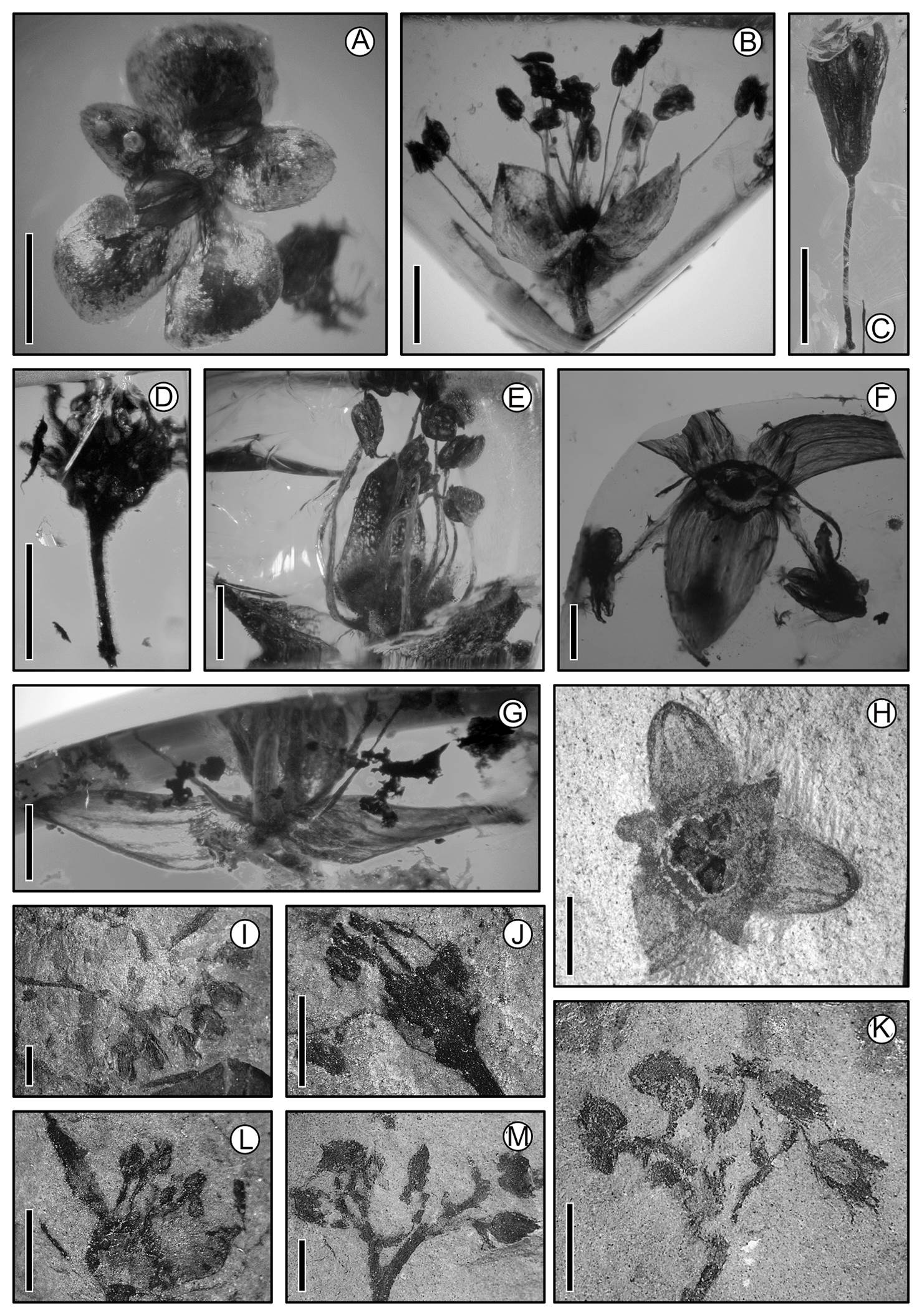
Figure 6 Flowers. (A)-(G) Miocene of Simojovel de Allende, Chiapas (15 Ma); (H) Oligocene, Tepexi de Rodríguez, Puebla (32 Ma); (I)-(K) Cretaceous, Carboniferous zone, Coahuila (70 Ma). (A) cf. Ulmaceae; scale = 1.0 mm. (B) Lunania floresi (Salicaceae); scale = 1. 0 mm. (C) Staphylea ochoterenae (Staphylaceae); scale = 1.5 mm. (D) Rhamnaceae-like flower; scale = 1.5 mm. (E) cf. Euphorbiaceae; scale = 1.0 mm. (F) cf. Arecaceae; scale = 0.5 mm. (G) cf. Butia (Arecaceae); scale = 1.0 mm. (H) Statzia; scale = 1.0 mm. (I) Inflorescence with scorpioid branching; scale = 0.05 mm. (J) Pedicellate flower with superior ovary and trimerous perianth; scale = 0.05. (K) Inflorescence; scale = 0.6 mm. (L) Flower with unguiculate petals and three stamens; scale = 0.5 mm. (M) Flowers with pentamerous perianth with calix and corolla; scale = 10 mm.
A striking example is the comparison of vegetation and flora established on the west coast of Mexico today and some 20 Ma ago. Today, tropical dry vegetation with marked seasonality is well established along the coast. Still, earlier, a tropical forest developed in more humid environments, possibly comparable with the humid forests of the East coast or even with vegetation types of tropical South America (e.g., Martínez-Cabrera & Cevallos Ferriz 2008). This situation highlights the importance of studying the outcrops of the last 135 Ma where angiosperms are important. The fossils also suggest that in the last 65 Ma, the so-called Boreotropical flora significantly influenced the formation of the distinct vegetation types in Mexico, and only later did the Neotropical flora complement our communities (e.g., Calvillo-Canadell & Cevallos-Ferriz 2005, Huerta Vergara & Cevallos-Ferriz 2018). Many elements of what is now called the Neotropical flora have exciting stories as part of the Boreotropical flora. The Miocene plants (15 to 20 Ma) from Simojovel de Allende, Chiapas, allow us to discuss this situation since lineages with an amphi-Atlantic distribution suggests closeness with African plants rather than South American ones (e.g., Hernández-Damián et al. 2019, 2021). Similarly, at least some Leguminosae, Anacardiaceae, and Humiriaceae taxa that were considered a contribution of the Neotropical flora began their history in Mexico ca. 50, 32 or 20 Ma ago, while the expansion of this flora towards the North is assumed to be more recent (e.g., Ramírez & Cevallos-Ferriz 2002, Calvillo-Canadell & Cevallos-Ferriz 2005, Flores-Rocha et al. 2013). This apparent ambiguity still needs to be studied and explained, highlighting the importance of studying Mexican fossil plants.
Mexican fossils can further contribute to the plant life histories. As described for the origin of roots, or the evolution of the conductive system and leaf differentiation for which information from the fossil record clarified important points of their histories, the extensive Cretaceous outcrops from Mexico provide the opportunity to contribute data from the past to reinforce the hypothesis on the origin of flowering plants. Furthermore, the origin of extant vegetation in Mexico will be enriched only by studying relatively recent deposits with Mexican fossils.
The fossils of Mexico and the studies carried out to date represent only slight indications of how we can investigate very different aspects of biology over geologic time, especially contributing to the understanding of the plants of the tropics in the past. Pending is the discovery and discussion of much of the biology of past times. Few Mexican specialists have had the opportunity to contribute to the understanding of life analyzed through time. It would be crucial that, in some way, researchers interested in the past life of different ages and geographic areas of the country could find opportunities to contribute to paleobotany. They can increase our understanding of the story that fossils of Mexico could tell and that, unfortunately still lies buried and awaiting new paleontological observations that will add information to different areas of biology and geology.











 nueva página del texto (beta)
nueva página del texto (beta)



