We dedicate this work to Efraím Hernández Xolocotzi, Arturo Gómez-Pompa,
Faustino Miranda, Jerzy Rzedowski, and José Sarukhán,
five pillars in the study of the vegetation of Mexico
Among all biological disciplines, ecology is one of the most widely embracing ones, as it covers an enormous range of research topics and subjects of study. A quick glance at general textbooks (e.g., Margalef 1977, Stiling 2002, Begon et al. 2005, Krebs 2009, Ghazoul 2019) or specialized journals in this field attests to this breadth. Therefore, reviewing the state-of-the-art in any ecological subdiscipline is always challenging, and this assertion certainly holds for vegetation ecology, the topic of this contribution. Vegetation ecology, a subdiscipline apparently first recognized as such by Mueller-Dombois & Ellenberg (1974) but undoubtedly with older foundations, is also a complex field of study, considering the various notions of vegetation emerging from (community ecology, population ecology, systems ecology, among others) (e.g., Braun-Blanquet 1972, Whittaker 1975, Archibold 1995, Terradas 2001, van der Maarel & Franklin 2013). In its broadest sense, vegetation is defined as the entire set of plants spontaneously established in a particular area on the planet (Box & Fujiwara 2013, van der Maarel & Franklin 2013). It is worth noting that this definition is silent about scales and thus it can be applied at different spatial and temporal scales. Though closely related, the concepts of vegetation and flora are quite different from each other; rather than on individual plants, the concept of flora focuses on the inventory of plant species occurring in one area (Lawrence 1951, Villaseñor & Meave 2022).
Vegetation ecology is a scientific discipline with a long-standing tradition but at the same time, it continues to be a very active and stimulating research area. To prove the validity of this contention, on October 2, 2021 we conducted a search in the Science Citation Index Expanded Database for research articles published between 2010 and 2020 that included the term ‘vegetation’ in the title, abstract, and/or keywords; the search produced 6,750 articles (yearly mean of 613.6, SD ± 76.5); the number of articles per year did not vary much over this period, with the lowest recorded in 2012 (530) and the highest in 2019 (747).
To identify the most active research areas currently addressed in vegetation ecology, we conducted a new search in the same database for articles published between January 1, 2010, and September 30, 2021, in the areas of Ecology, Forestry and Plant Sciences that included the term ‘vegetation’, along with other 17 research topics, either in the title, abstract, or keywords (Figure 1). To assess how the frequency of these research topics changed when performing this analysis for a specialized journal, we repeated this search but limiting it to two specialized periodicals: Journal of Vegetation Science and Plant Ecology. The topics with the three highest ranks according to both search strategies were diversity, composition, and structure, while other topics such as conservation, management, restoration, and succession also stood out (Figure 1). In this review, we focus on those research areas having the highest frequencies in Figure 1, encompassing six main topics. In doing this, we examine some current challenges and research perspectives in vegetation ecology for the future.
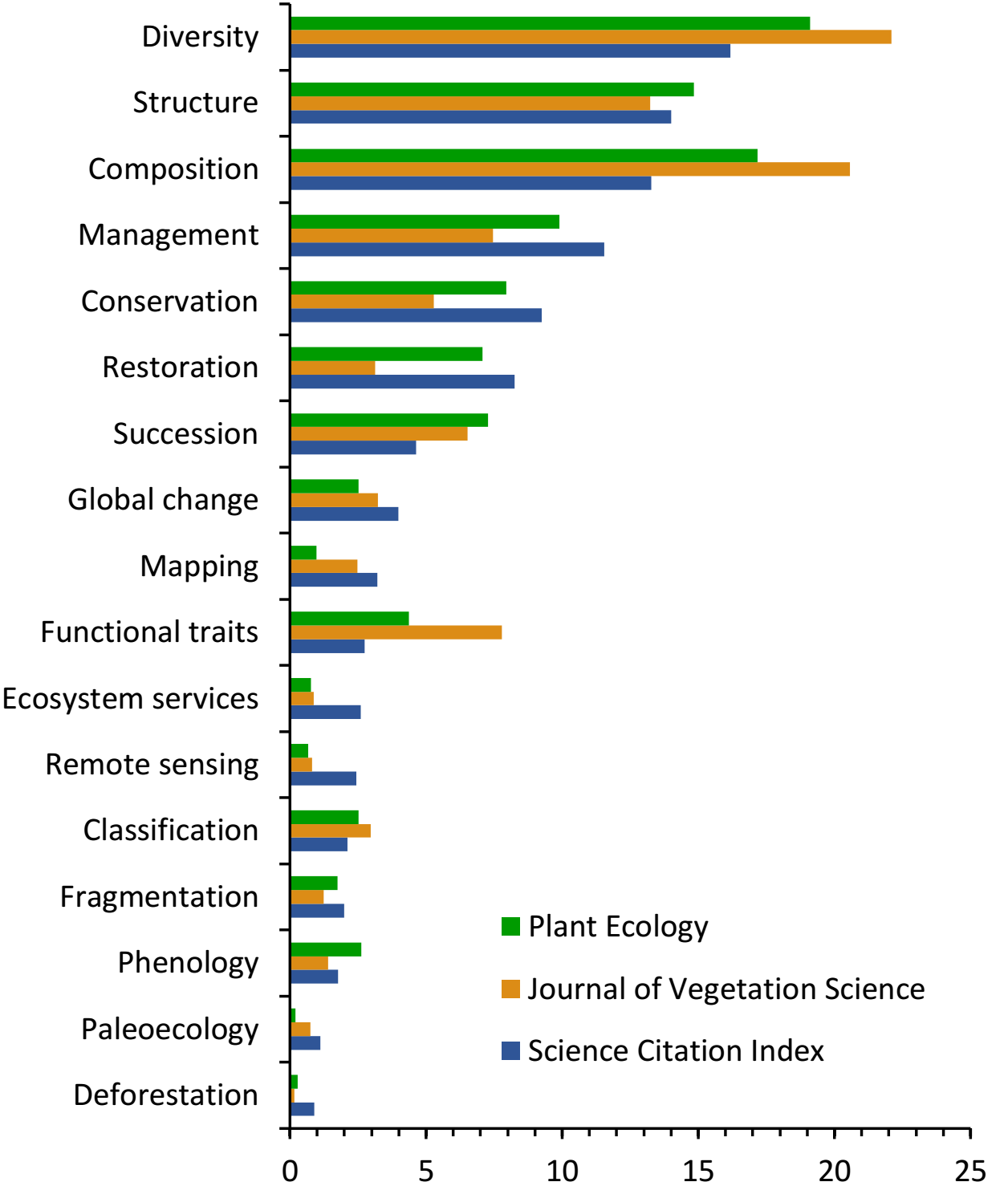
Figure 1 Proportion of research articles retrieved from the Science Citation Index Expanded (SCI) database having in the title, abstract, and/or keywords the term Vegetation in combination with any of the 17 topics shown. In total, these 17 combinations resulted in 14,891 papers, whereas 1,069 and 1,917 articles corresponded to the specialized journals Plant Ecology and Journal of Vegetation Science, respectively. Research topics are plotted in decreasing order according to their proportion of the total number of items retrieved in the SCI.
In preparing this review, biases derived from the particular fields or approaches practiced by the authors were inevitable. For example, the readers will notice that many examples and ideas are related to forest communities, often tropical and subtropical. This should not be interpreted as an indication of a despising attitude towards non-forest plant communities or those typical of other climatic regions, but rather as a reflection of our limited experience with them, ultimately resulting in the risk of making inadequate judgments. In our defense, we can only say that we hope that this review will stimulate similar efforts directed with higher precision at the different community types that comprise the extremely complex and varied vegetation cover of our planet.
Vegetation description and characterization
The three-dimensional structure of plant communities. Understanding and explaining vegetation attributes in a specific space and time is a challenging endeavor, as it requires the examination of environmental and biological factors that have a very dynamic nature both in space and time (Pan et al. 2013, Peet & Roberts 2013, Hédl et al. 2017, Birks 2019). The recognition of vegetation formations or similar vegetation categories has the strongest foundations in its floristic composition, and structural and functional attributes such as dominant growth forms, canopy height, or leaf phenology (Rzedowski 1978, Keith & Pellow 2015, Mucina et al. 2016, Guo et al. 2018, Mengist 2019). Examination of these vegetation attributes has helped strengthen and clarify the delimitation of vegetation types previously distinguished based on their physiognomy only (Sánchez-Rodríguez et al. 2003, Fashing & Gathua 2004, Castillo-Campos et al. 2008, Ramesh et al. 2010, Dexter et al. 2015, Astudillo-Sánchez et al. 2019, Hurtado-Reveles et al. 2021).
In order to meet multiple needs and take advantage of available resources, a wide array of field methods have been developed to quantitatively assess the different vegetation attributes (e.g., Kershaw 1973, Mueller-Dombois & Ellenberg 1974, Greig-Smith 1983, Kent 2012, Arellano et al. 2016). Similarly, numerous techniques can be used to represent individual plants, populations, communities, and groups of communities across landscapes and biomes (Pedrotti 2013, Faber-Langendoen et al. 2014). Nonetheless, these methods are not always rigorously applied in vegetation sampling, and basic methodological details are often missing in scientific publications. This issue becomes critical in tree size assessment or biomass estimations. In forest communities, the most widely agreed upon option is to measure the diameter at breast height (DBH). However, because the trunk of many trees, especially in tropical forests, is wider near its base, the height at which their diameter is measured influences the recorded value. Brokaw & Thompson (2000) found that the breast height is often not explicitly defined and that the height at which it is measured ranges from 1.2 to 1.6 m; to avoid inconsistencies, they recommend always setting the BH at 1.3 m. Yet, deciding on a unified trunk diameter measurement is not straightforward when trees have buttresses, aerial roots, thorns, spines, or prickles, when their trunks are crooked or bent, when individuals to be measured grow on steep slopes, or if they have very thick trunks (Figure 2). Each of these situations requires specific solutions, and these should always be fully disclosed (e.g., Phillips et al. 2016, Moonlight et al. 2021). For example, the diameter of a tree with buttresses should be measured with calipers above these structures, where the trunk becomes more uniform. Lianas, a growth form particularly prominent in wet tropical forests (Schnitzer & Bongers 2002, Solórzano et al. 2002) are not exempted from these measuring problems (Figure 3), and Gerwing et al. (2006) recommended measuring the stem diameter for these plants 1.3 m from their main rooting positions.
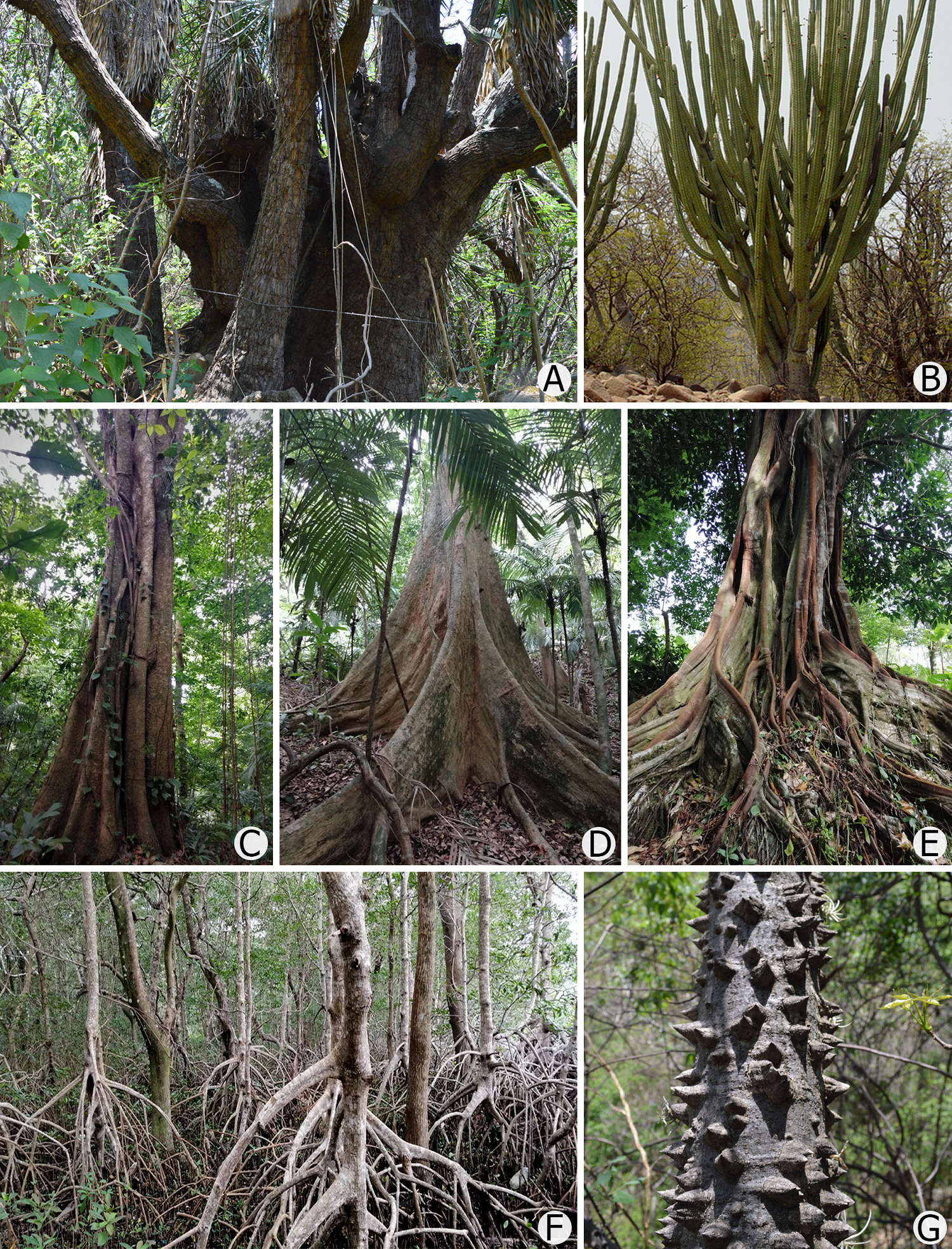
Figure 2 Examples of plants for which measuring diameter at breast height (DBH; 1.3 m) is problematic. (A) Yucca filifera Chabaud (Asparagaceae), with a very wide trunk near its base. (B) Stenocereus quevedonis (J.G. Ortega) Buxb. (Cactaceae), with a very short main trunk. (C) Mortoniodendron guatemalense Standl. & Steyerm. (Malvaceae), species with a characteristic ribbed trunk. (D) Dussia mexicana (Standl.) Harms, (Fabaceae), tree with very tall (up to 6 m) buttresses. (E) Ficus isophlebia Standl. (Moraceae), strangler-tree whose "trunk" is made up of numerous aerial roots; the proper trunk is located several meters above the ground. (F) Rhizophora mangle L. (Rhizophoraceae) with stilt roots arising from the trunk. (G) Ceiba aesculifolia (Kunth) Britten & Baker f. (Malvaceae), young trunk densely covered with prickles.
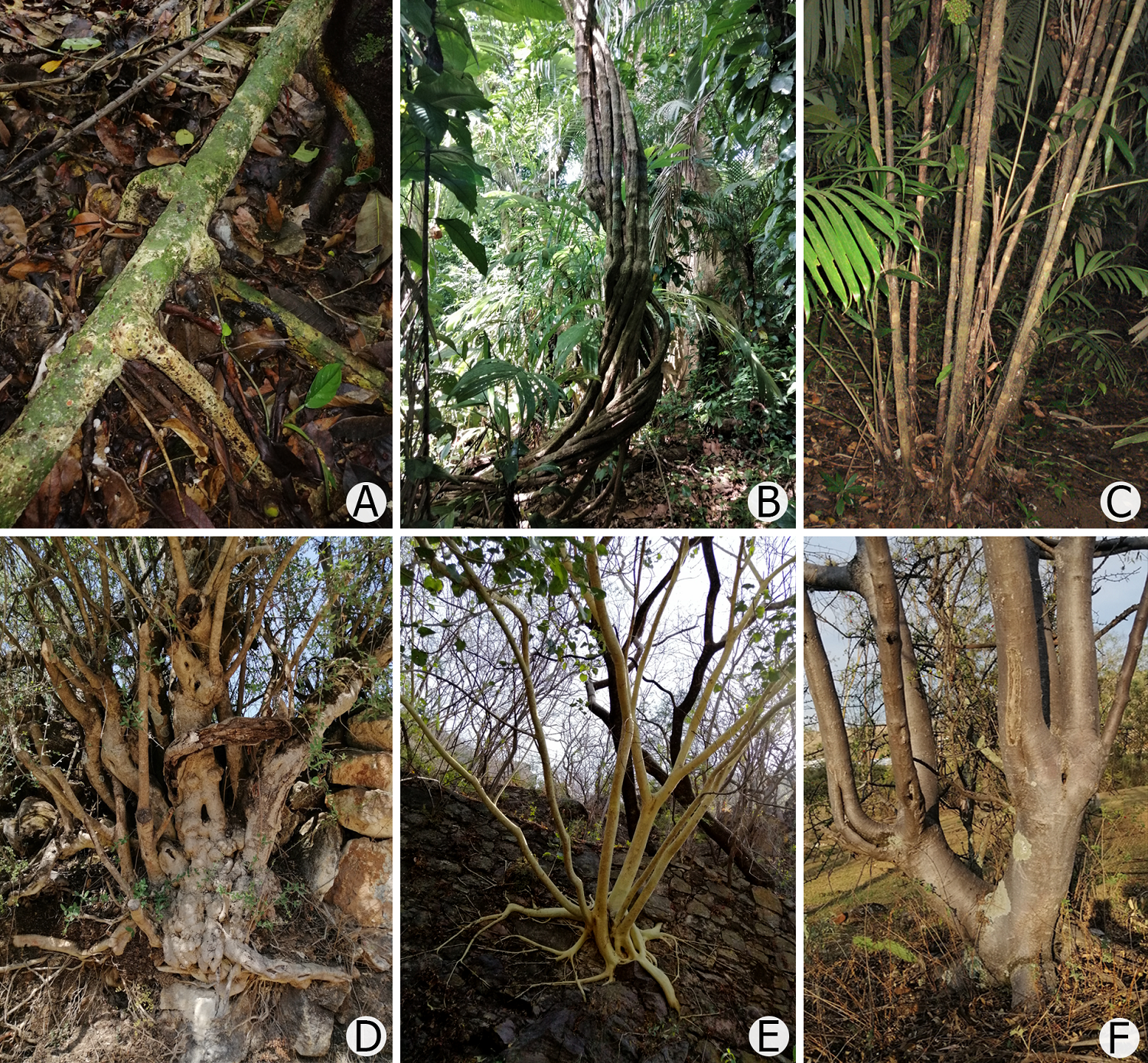
Figure 3 Examples of multi-stemmed plants for which different criteria may be applied to measure their stems, which may result in different morphometric or biomass variable estimations. (A) Callichlamys latifolia (Rich.) K. Schum. (Bignoniaceae), liana with several rooting points. (B) Mansoa verrucifera (Schltdl.) A.H. Gentry (Bignoniaceae), liana with multiple rooting positions and stems. (C) Bactris mexicana Mart. (Arecaceae), understory palm whose stems have a dense cover of spines. (D) Forestiera phillyreoides (Benth.) Torr. (Oleaceae), short tree with multiple stems growing very close to each other. (E) Ficus petiolaris Kunth (Moraceae), young tree growing a rocky substrate with multiple leaning stems. (F) Bursera cuneata (Schltdl.) Engl. (Burseraceae), tree with one stem growing upright and another one leaning.
In describing forest structure based on stem diameter measurements, multi-stemmed plants are particularly problematic (Figure 3). Multi-stemmed trees abound in tropical dry forests (Dunphy et al. 2000, Ramos et al. 2020) but are frequent in other forest types as well, particularly under limiting conditions for plant growth such as low soil fertility (Bellingham & Sparrow 2009), chronic disturbance (Nzunda et al. 2007) or strong interspecific competition (Tanentzap et al. 2012). Therefore, the question of how to properly measure these trees has been a permanent concern in the minds of vegetation ecologists (Magarik et al. 2020, Moonlight et al. 2021). Despite the earlier suggestion to measure stem diameter below BH as a solution (Rodal et al. 2008), this approach has the disadvantage of affecting among-community comparability. When dealing with multi-stemmed trees, the true problem arises when deciding whether to measure all stems of a tree in which only one or few stems meet an a priori sampling criterion. This issue was analyzed by de Souza et al. (2021) through a comparison of the by stem vs. by tree methods to decide the inclusion of a tree in the sampling. These authors showed that the by-stem method excludes many stems, resulting in considerable underestimation of community variables like basal area and aboveground biomass compared with the by-tree method (the latter method can add trees to the sample when their combined diameters are equivalent to the minimum threshold established for the sampling). Future forest inventory and monitoring programs will have to pay attention to these caveats to make the best decisions to increase the results comparability.
The proportion of a forest canopy becoming leafless in the unfavorable season is a common criterion for vegetation description and classification (Rzedowski 1978, FAO 2012, Guo et al. 2018, Mengist 2019, Muldavin et al. 2021). However, this attribute is highly dynamic in time and space, making its assessment problematic. For example, in the case of tropical forests, the deciduous fraction of the canopy should be quantified at the peak of the rainless season, while assessing this attribute in the rainy season should be avoided. Moreover, leaf phenology as input for forest classification is usually assessed without a defined spatial reference, using increasing proportions of foliage retention based on visual appraisal (e.g., from evergreen [75-100 % of leafy trees] to deciduous forests [0-25 % of leafy trees]). Information on leaf phenology over several annual cycles may also be useful to solve the problems that hinder the accurate characterization of some vegetation types. In this context, remote sensing offers a promising alternative to improving community-level assessment of leaf phenology and its variation throughout the year (Kalácska et al. 2007, Hesketh & Sanchez-Azofeifa 2014, Cavender-Bares et al. 2020) as, contrary to field-based methods, it allows for complete spatial analyses of the Earth’s surface more rapidly.
Maximum tree height in a community as a proxy to canopy height -and thus community development- is commonly used to distinguish vegetation types (Rzedowski 1978, FAO 2012, Guo et al. 2018, Mengist 2019, Muldavin et al. 2021). However, accurately measuring this attribute also faces various problems. Although there are instruments that facilitate this task (e.g., clinometers or rangefinder/hypsometers), tree height measurement relies more often on visual estimations (Sánchez-Rodríguez et al. 2003, Ramesh et al. 2010). The size of the errors derived from this practice is worrisome, considering that tree height is a variable frequently used in allometry-based biomass estimation (Chave et al. 2015, Bojórquez et al. 2020). When quantitative vegetation sampling has been conducted, mean canopy height can be estimated from the heights of upper stratum trees, either using the total number of individuals, the uppermost decile (10 %) of the tallest trees, or the 10 tallest individuals (Salas-Morales et al. 2018, Chávez et al. 2020); alternatively, the heights of a standardized number of individuals (e.g., > 50 individuals with DBH > 10 cm in tropical forests) from the upper canopy can be averaged (Torello-Raventos et al. 2013).
Recently, the challenge of spatial and temporal limitation of vegetation height data has been tackled with the LiDAR (light detection and ranging) remote sensing technology. LiDAR can create a detailed three-dimensional reconstruction of vegetation, including those portions below the upper canopy (Popescu et al. 2002, Castillo-Núñez et al. 2011, Wulder et al. 2012, Guo et al. 2017). Another advantage of using LiDAR is that the resulting vegetation mapping can be clearly related to various topographic terrain attributes while obtaining valuable information on other structural variables (e.g., tree density or basal area) or on vegetation dynamics (Heurich 2008, Castillo-Núñez et al. 2011, Wulder et al. 2012, Lausch et al. 2020). However, decisions on the method to be used and the choice of parameters to be assessed to describe canopy structure or any other attribute of the plant community of interest should always be governed by a clear definition of the ecological questions being asked (Bongers 2001).
The tribulations of assessing plant community diversity. A fundamental question that has long stimulated ecological research is related to the factors involved in the unequal distribution of species richness on Earth. This debate is marked by the recognition of a well-known latitudinal pattern reflected as higher species richness of tropical vegetation than their counterparts of other latitudes (Willig & Presley 2018, Pontarp et al. 2019), a pattern that depends on the taxonomic category and spatial scale (Willig et al. 2003). Gentry (1988) and Ellison (2002) provided significant evidence of the negative link between plant richness and latitude, with the opposite pattern found at smaller spatial scales (Becerra 2016, Behera & Roy 2019), for aquatic plants (Willig et al. 2003) and some temperate tree genera such as Pinus (Pinaceae) and Quercus (Fagaceae) (Arenas-Navarro et al. 2020, Vega et al. 2020).
It is important to note, however, that downscaling the assessment of the floristic richness at regional, subcontinental, and continental scales to the plant community level is not straightforward, as it requires moving from large-scale biodiversity inventories to local diversity assessment. Evaluating and comparing the numbers of species occurring locally in vegetation is often achieved through vegetation sampling (Mueller-Dombois & Ellenberg 1974, Kent 2012). A very popular method for this purpose was proposed by Gentry (1982), mainly used in tropical forests, which consists of the recording of woody plants in areas of 0.1 ha dissected in ten 50 ( 2 m transects. Data gathered with this method have provided ample base for understanding the distribution of plant richness and its underlying factors, mainly total annual rainfall and potential evapotranspiration in the case of tropical forests (Phillips & Miller 2002, Trejo & Dirzo 2002).
Quantitative vegetation sampling demands financial, material, and time resources, all of which usually are in short supply; consequently, large-scale vegetation inventories are much scarcer than sampling carried out in small areas. This is unfortunate, as the use of large plots to study vegetation usually results in new, unforeseen findings. For example, in temperate forests of both eastern and western North America, maximum tree height is positively associated with tree species richness and local diversity, but this relationship is weakened by environmental harshness (Marks et al. 2016). In tropical forests, censuses of 1 to 50 (occasionally up to 130) ha have allowed recording rare species, making more predictive species-area curves, and evaluating the extent to which plant abundance and distribution patterns are explained by different environmental factors (e.g., topographical heterogeneity or climate), random drift, or dispersal limitations (Bongers et al. 1988, Meave et al. 1992, Fangliang et al. 1997, Harms et al. 2001, Small et al. 2004, Valencia et al. 2004, Poulsen et al. 2006, Krishnamurthy et al. 2010, Harris et al. 2020). In this regard, two relevant findings derived from the evaluation of species richness in tropical forests can be highlighted (Condit et al. 1996): (1) the species-area accumulation curve did not show an asymptote at 50 ha, and (2) in evaluating between-forest differences, species richness should not be used as the only diversity estimator if fewer than 1,000 stems are sampled.
This latter recommendation points to the importance of assessing plant community diversity in a comprehensive manner, combining species richness (the number of species in the system of study) and its evenness (the variation in the relative abundances of all species occurring in the community). During the second half of the 20th century, most studies combined richness and evenness into diversity indices (e.g., the Shannon entropy index or the Gini-Simpson index). However, the validity of this practice has been questioned because the values (and units) of these indices are not comparable with each other, and also because they do not fulfill mathematical properties such as the replication principle or ‘doubling property’ (Jost 2006, 2010). This has led to invalid inferences from an ecological standpoint. Therefore, at present there is an increasing trend to use true diversity measures based on Hill numbers (Hill 1973), as these diversity measures allow a direct comparison of diversity between two or more communities based on a unified diversity unit, namely the ‘effective number of species’ (Jost 2006, Moreno et al. 2011). Within this framework, the diversity order value q is related to species rarity or abundance; though a continuous variable, usually only three values of q are used in current diversity analyses (q = 0, 1, and 2): (1) 0 D, which provides a diversity estimate insensitive to the relative abundances of species and thus best represents total species richness, (2) 1 D, which corresponds to the case in which the species differential abundances are considered and thus roughly indicates the number of common species, and (3) 2 D, which allows giving more weight to the most abundant species and thus roughly represents the number of dominant species.
Vegetation-environment relationships: a tough nut to crack
If there is any single feature characterizing the vegetation cover across all continents, it is its enormous variability. For centuries, scholars from many disciplines, but most prominently from Botany, have sought to find the major underlying relationships between the multiple manifestations of the Earth’s vegetation and their environmental drivers. Within this framework, perhaps the most significant early contribution is the work of de Humboldt and Bonpland (1805), in which they described with stunning detail vegetational changes along the slopes of the South American Chimborazo volcano, at the time believed to be the tallest mountain on the planet. Thereafter, the number of published works on this subject has been plethoric. Thanks to such profuse activity, during the 20th century and by the early 21st century, the main relationships between broad vegetation units and major climatic regions became well established (Walter 1973, Mueller-Dombois & Ellenberg 1974, Whittaker 1975, 1978, Woodward & McKee 1991, Archibold 1995, Terradas 2001, Lapola et al. 2008).
However, no vegetation ecologist would be ready to chant victory on this front. This is so because the seemingly infinite vegetation variability is not only influenced by macro-climatic conditions; quite on the contrary, there is increasing evidence for the crucial roles of a multitude of smaller-scale environmental factors, including microclimatic, topographic, and edaphic, as well as a large array of natural and anthropic disturbances, all determining the variable composition and structure of plant communities (Gentry 1988, Ibarra-Manríquez & Martínez-Ramos 2002, DeWalt et al. 2010, Do et al. 2015, Marks et al. 2016, Méndez-Toribio et al. 2016, 2020, Bueno et al. 2018, Arévalo et al. 2021, Feng et al. 2021, Sánchez-Reyes et al. 2021).
The effects of microclimate on vegetation attributes can be strongly modified or even overrun by the characteristics of the substrate on which each plant community develops. For example, several studies have highlighted the distinctly different attributes of tropical vegetation on limestone bedrock, which often evolves into a karstic terrain, emphasizing their contrasts with communities typical of other substrates (Ibarra-Manríquez & Martínez-Ramos 2002, Pérez-García & Meave 2005, Pérez-García et al. 2009, Coelho et al. 2013, Navarrete-Segueda et al. 2017, Geekiyanage et al. 2019, Esparza-Olguín & Martínez-Romero 2021), although the effect of rocky outcrops has been also reported for tropical mountain vegetation, even in very moist habitats (e.g., Williams-Linera & Vizcaíno-Bravo 2016).
Recently, much research has focused on the role of small-scale substrate or geomorphological variation in determining the heterogeneity of vegetation attributes. In a tropical rainforest, Poulsen et al. (2006) found a large variability in the physical and chemical soil properties that determined the distribution of various plant groups within a 1-ha plot. More recently, de Souza et al. (2020) demonstrated that small-scale edaphic heterogeneity is reflected in the spatial variation of tropical dry forest structure.
Topography is a further source of vegetational variation at small and medium scales within a region, even under relatively homogeneous climatic conditions. The different topographic units are associated with relevant variation in edaphic factors, which results in critical differences in soil fertility, water availability, energy budgets, and in general, growing conditions for plants (Segura et al. 2002, Gallardo-Cruz et al. 2009, Méndez-Toribio et al. 2016, Navarrete-Segueda et al. 2018). In some instances, these findings have unsuspected implications. For example, in examining the variation of aboveground biomass in a subtropical moist forest in China, Xu et al. (2015) found that this variable was mainly related to the density of large trees, which in turn was finely associated with topographic heterogeneity. Results like this point to the urgent need to always associate measures of error to forest attribute estimates as important as biomass (both above- and belowground) to understand the climatic regulation of forests. The situation becomes more complex when the topographic heterogeneity interacts with climatic variability, particularly along rainfall gradients, as the different topographic units interact synergistically with the amount of rainfall water received in the different sections of the landscape (Muscarella et al. 2020). These complex interactions are not easy to disentangle, but it is worth making bigger efforts in this line of research, as it will allow us to better understand landscape (meso-) scale vegetational mosaics superimposed on substrate-related environmental mixtures (Durán et al. 2006, Gallardo-Cruz et al. 2010; Pérez-García et al. 2010, Block & Meave 2017) and to guide potential management and conservation applications for them (Zerbe 1998, Navarrete-Segueda et al. 2017, 2021).
One interesting aspect revealed by the investigation of the role of geomorphology in determining vegetation heterogeneity is that very small-scale geomorphological variation (namely, differences of a few meters or even centimeters between high and low topographic positions) is sufficient to create considerably high local vegetation heterogeneity. This is particularly evident in plant communities subjected to complex flooding regimes, where such differences are often associated with flooding duration or depth (Chávez et al. 2020, Solórzano et al. 2020), but there are also examples of alpine vegetation (Opedal et al. 2015).
Vegetation ecologists have been fascinated since the dawn of the discipline by mountains and their vegetation. The study of vegetation and its changes along elevational gradients remains not only a popular but also a challenging research topic in vegetation ecology (Willig et al. 2003, Sundqvist et al. 2013) since these gradients can be used as research systems to understand vegetation responses to environmental changes. Vegetation compositional and structural heterogeneity related to elevational gradients has been repeatedly analyzed for tree species, usually on a single gradient (e.g., Vázquez G & Givnish 1998, Kappelle & van Uffelen 2006, Acharya et al. 2011, Salas-Morales & Meave 2012, Maza-Villalobos et al. 2014, Becerra 2016, Cui & Zheng 2016, Salas-Morales & Williams-Linera 2019; but see Martínez-Camilo et al. 2018, and Zhao et al. 2022, who included several replicated parallel elevational gradients); yet information for other growth forms is still scanty (Sánchez-González & López-Mata 2005, Gómez-Díaz et al. 2017, Rascón-Ayala et al. 2018, Cirimwami et al. 2019, Guzmán-Jacob et al. 2020, Ohdo & Takahashi 2020, Xu et al. 2021, Zhao et al. 2022).
Some factors such as atmospheric pressure, temperature, and solar irradiance covary with elevation and can be related to other environmental variables such as precipitation, soil attributes, and geology (e.g., Ramos et al. 2020), but temperature and land area emerge as the most important factors explaining changes in the biota along elevational gradients (Körner 2007, Sundqvist et al. 2013). As in the case of horizontal environmental variation, new insights into the variation of vegetation structure along elevational gradients can be gained from a spatially high-resolution assessment of environmental variation, including microclimatic conditions (e.g., Salas-Morales et al. 2015).
Vegetation classification: getting the big picture
Due to the difficulty of recognizing and delimiting different vegetation classes, to this date no universally accepted classification scheme exists (De Cáceres & Wiser 2012, Faber-Langendoen et al. 2014). In addition, for vegetation types occurring in more than one region or continent, comparisons can be even more difficult since they are usually named using different terminology even when sharing similar structural attributes (Torello-Raventos et al. 2013). However, if a single, universal vegetation classification were available, this would have positive impacts in different fields of biology, for instance, in ecological studies, restoration strategies, conservation biology, biogeographical regionalization, or natural resource management.
Several authors have reviewed the historical development of different vegetation classification systems (Mueller-Dombois & Ellenberg 1974, Rzedowski 1978, Box & Fujiwara 2013, Faber-Langendoen et al. 2014, De Cáceres et al. 2015, Velázquez et al. 2016, Cámara Artigas et al. 2020, Nunes et al. 2020). Globally, various categories to classify the vegetation have been proposed, whose equivalence (both nomenclatural and practical) is not always possible. One of the most frequent categories in the literature is ‘vegetation formation type’, which can be present in different continents (e.g., mangroves or tundra). By contrast, more spatially-restricted, regional vegetation units in a particular continent (‘formations’) are recognized by their structural and physiognomic uniformity, although their floristic composition usually varies widely between regions (Box & Fujiwara 2013). Another category amply used is biome, defined by Pennington et al. (2018, p. 541) as: “... major vegetation formations with distinct physical forms (physiognomies) and ecological processes that can be characterised at a global scale”.
Vegetation formations are defined by qualitative and/or quantitative attributes related to intrinsic properties including floristic composition, the dominance of some taxa, diversity, physiognomy, structure, leaf phenology, or also properties extrinsic to vegetation such as environmental factors (e.g., climate or soil), habitat, or region in which vegetation occurs (Miranda & Hernández-X. 1963, Greenway 1973, Mueller-Dombois & Ellenberg 1974, FAO 2012, Box & Fujiwara 2013, van der Maarel & Franklin 2013, Faber-Langendoen et al. 2014, Meave et al. 2016, Mucina et al. 2016, Guo et al. 2018, Mengist 2019, Muldavin et al. 2021). Researchers have continued to pursue the goal to provide vegetation classifications on a global scale in the last decade; in these efforts, more detailed information on those factors strongly associated with its distribution has been incorporated, such as the interpolation of climate and elevation variables (Pan et al. 2013, De Cáceres et al. 2015, Fick & Hijmans 2017, Cámara Artigas et al. 2020). Vegetation classifications have also incorporated increasingly detailed cartographic information, as well as satellite imagery, to determine temporal and spatial changes in vegetation and have benefited from the availability of more powerful software to perform data analysis (Hernández-Stefanoni et al. 2012, Pan et al. 2013, INEGI 2015, Leitão et al. 2015, Sanchez-Azofeifa et al. 2017, Zhang et al. 2017, Alleaume et al. 2018, Watanabe et al. 2020, Petropavlovsky & Varchenko 2021). A few examples of these proposals will be briefly examined below.
The United Nations Food and Agriculture Organization (FAO) makes periodic contributions to the Global Forest Ecological Zones (GEZ) to map and characterize different forest types, with names that can be understood and used worldwide (FAO 2012). The proposal includes 20 GEZs (e.g., boreal tundra woodland, subtropical desert, or tropical dry forest), associating a map to each climatic group and providing the equivalence of the GEZs to vegetation classifications in different regions of the world. In turn, Faber-Langendoen et al. (2014) designed a classification system (EcoVeg) for natural or cultural (i.e., with strong human influence) vegetation, integrating physiognomic aspects (growth forms and structure), floristic composition, and ecological attributes (e.g., bioclimatic variables, biogeographic affinities, degree of disturbance, and soil types). This proposal aims at characterizing vegetation types in eight hierarchical levels, subdivided in: (1) three upper ones (based on the formations criterion, on a global or continental scale), (2) three intermediate ones (named divisions and macrogroups, which combine species based on their biogeographic origin and ecological factors considered at a regional scale), and (3) two lower ones (i.e., alliance and associations, whose definition considers the composition, habitat, physiognomy, diagnostic species and disturbance gradients at local to regional scales). The criteria underlying this classification system are explained in detail, with examples for some regions of the Earth.
Another promising system worth looking at is the IUCN Global Ecosystem Typology, which is a hierarchical classification system of the ecosystems, which are recognized by their ecological functions and species composition (Keith et al. 2020). The upper levels in this system are five realms. The terrestrial realm includes all dry land, divided into seven biomes, which, in turn, encompass 34 ecosystem functional groups (a category somewhat equivalent to ‘vegetation formation type’).
In classifying vegetation at different scales, it must be noted that local or regional classification schemes are often quite different in their scope of the vegetation type concept from worldwide classifications. Small-scale classifications often have at hand detailed information on the plant communities occurring in the focal region, both for vegetation structure and floristic composition, and this allows fine-tuning the vegetational categories recognized in the system. Yet this task may encounter difficulties when the vegetation to be classified is spread across national boundaries. This situation is exemplified by the vegetation classification for the Usumacinta River Basin (Meave et al. 2021), which includes parts of the territories of neighboring Guatemala and Mexico, and which does not fully match any previous vegetation classification scheme for either country separately.
You cannot step into the same river twice: analyzing vegetation from a dynamic perspective
Plants depend on essential resources such as solar energy, carbon dioxide, water, and mineral nutrients to survive, grow, and reproduce. Plants living in a given habitat exhibit adaptations to capture and use available resources, cope with the prevailing abiotic conditions, and interact with species of the same and other trophic levels. Resources, conditions, and interactions change in nature and magnitude over time in the same locality and across space, affecting plant species differentially. As a result, vegetation abundance, diversity, and composition change within and across localities. All these changes can be encompassed under the term “vegetation dynamics” (Pickett et al. 2013).
Long-term dynamics. Understanding the movement of tectonic plates (continental drift) of the planet helps explain historical causes determining the species composition of contemporary vegetation (Chaboureau et al. 2014). For example, coniferous forests in cool regions of Australia, New Zealand, Antarctica, and South America (Chile and Argentina) are dominated by closely related species in the Araucariaceae and Podocarpaceae families. More than 200 million years ago, these regions were part of the great Gondwana continent (Kershaw & Wagstaff 2001), specifically of the so-called Weddelian Biogeographic Province, where those conifer groups evolved (Dutra 2004). The vegetation in these regions preserved part of this historical legacy as the tectonic plates separated, forming new continents. However, the arrival of angiosperm plants, whose evolution is estimated to have begun more than 120 million years ago (Chaboureau et al. 2014), enriched the vegetation of each region differentially. Historical traces in the current vegetation composition, associated with continental drift, can be found throughout the Earth’s crust (Graham 1999, Morley 2003, Cevallos-Ferriz & González-Torres 2006); for instance, the biogeographical differentiation of Neotropical secondary forests across the Americas seems to have withstood so far the homogenizing effect of land use change through agricultural development, which in theory promotes the geographical development of pioneer species with high dispersal abilities (Jakovac et al. 2022).
The methods used to understand historical changes in vegetation are often based on fossilized pollen. This pollen occurs in sediment cores extracted from lacustrine and marine systems whose anoxic conditions favor the long-term preservation of the pollen deposited over time (Prentice 1988, Willis et al. 2010). Pollen found in deeper core sections came from plants older than plants whose pollen is found in the upper core sections. In addition, sediments can be dated through radioisotopic techniques (Björck & Wohlfarth 2002). With this basis, it has been documented that vegetation undergoes dynamic changes (in timeframes of thousands of years) in its structure and composition, associated with variations in climate, fire regimes, and human activity. For example, on the Atlantic coast of Brazil savanna vegetation dominated during a 9,000-year arid period, with high fire frequency, while gallery forests expanded in periods of high rainfall and low fire frequency. These changes interacted with topography: on the hills, open dry forest shifted to semi-closed forest between dry and wet periods, respectively (Behling 2003). Likewise, in Madagascar the littoral forest was replaced within a 6,000-year period by scrub vegetation due to increasing aridity and rising sea level (Virah-Sawmy et al. 2009), and in the Sierra de Manantlán, Mexico, in arid periods the dominant vegetation for over 4,000 years was a pine forest that replaced cloud forest, the dominant vegetation in humid periods (Figueroa-Rangel et al. 2008). Over 5,000 years, the diversity of plant species in the dry tropical forest of western Mexico has markedly dwindled during periods of severe drought associated with the El Niño phenomenon (Lozano-García et al. 2021). In addition, long-term studies based on paleo-records have allowed the analysis of changes in ecological attributes (resilience, vulnerability, stability, niche separation, life history strategies, as well as contrasts between environmental determinism and neutrality, among other topics) in plant communities associated with historical natural or human-induced disturbances (Willis et al. 2010, Correa-Metrio et al. 2014, Islebe et al. 2018).
Medium-term dynamics. Vegetation is exposed to sudden environmental changes caused by disturbances. Disturbances can be physical, chemical, or biotic agents that destroy vegetation totally or partially. Disturbance regimes can be characterized by disturbance extent, magnitude, frequency, and duration (Pickett & White 1985). Disturbances open spaces where a process of species replacement and vegetation development begins, a process that we know as (ecological) succession (Gibson & Brown 1985). Succession may or may not result in plant communities similar to those found before the disturbance (Chazdon 2014).
Succession is an important process in determining vegetation dynamics. When the disturbances cause a perturbation (the state displayed by the ecosystem after the disturbance) of high magnitude, leaving a sterile substrate, the so-called primary succession begins, although there has been discussion about the usefulness of this term (van Andel et al. 1993). Among those disturbances triggering primary succession are volcanic eruptions, lateral migration of rivers on floodplains, retreating glaciers, and rising coastlines (Laliberté & Payette 2008). Primary succession starts with the arrival of spores and seeds from external sources. Other disturbances such as hurricanes, fires, landslides, earthquakes, tsunamis, and natural treefalls do not cause the total loss of the biota. The remaining organisms in the disturbed site initiate the so-called secondary succession, although propagules that arrive from external sources (transported by animals or the wind) also participate (Horn 1974, Chazdon 2014, Arroyo-Rodríguez et al. 2017).
Succession can last from tens to hundreds of years, depending on the disturbance regime and species’ life histories. Long-lasting succession occurs when the pre-disturbance vegetation includes slow-growing, long-lived plants. For example, forests in temperate and tropical regions are often made up of trees whose longevities range from several hundred to a few thousand years (Martínez-Ramos & Álvarez-Buylla 1998, Piovesan & Biondi 2021).
A direct way to study plant succession is the monitoring of vegetation changes over time in permanent plots established in recently disturbed sites (Foster & Tilman 2000). This approach is known as longitudinal or dynamic. However, for succession processes that last several tens or hundreds of years, it is difficult to cover the entire succession process with this approach, although some longitudinal studies have followed plant succession for more than 25 years (e.g., Kardol et al. 2010, Martínez-Ramos et al. 2021b, Prach et al. 2021). This long-term longitudinal approach has enabled new analyses, for example, the role of autogenic regulation in tropical forest resilience (Muñoz et al. 2021).
Another widely used approach for studying succession is the chronosequence method, in which time is replaced by space (Walker et al. 2010). In a given locality, sites with different ages since disturbance cessation (i.e., successional age) are selected, ideally including recently, intermediate, and old disturbed sites. Successional age can be obtained from landowners (e.g., van Breugel et al. 2006) or estimated using dating tools such as tree growth rings (Abrams et al. 1995, Brienen et al. 2009) or aerial photographs and satellite images (Whited et al. 2007, Gallardo-Cruz et al. 2012, Sánchez-Reyes et al. 2017, Abbas et al. 2020). At each site, a plot with an area defined according to the vegetation and the study objectives is established to record vegetation attributes (e.g., abundance, biomass, diversity, and species composition). By relating these attributes to successional age, predicted trajectories of vegetation change during succession can be obtained (e.g., Chazdon et al. 2007, Lebrija-Trejos et al. 2010, Mora et al. 2015).
The chronosequence method has in its favor the advantage of allowing a rapid reconstruction of the long-term successional processes. However, it has the disadvantage of assuming that the environmental conditions prevailing at the beginning and during succession were the same at all sites included in the chronosequence (Foster & Tilman 2000). This assumption may be unrealistic is unrealistic because different environmental factors, external to successional age, vary among seasons and years from site to site (Johnson & Miyanishi 2008). Thus, the state of the climate, the abiotic conditions of the substrate, the biota within and around the disturbed sites, and the extent and magnitude of disturbance may vary among sites disturbed in different years (Álvarez-Yépiz et al. 2008, Martínez-Ramos et al. 2018, Pérez-Cárdenas et al. 2021). To reduce this problem, chronosequences can be constructed with sites as similar as possible in soil characteristics, topography, surrounding matrix, and disturbance histories, or with a high replication level (Dupuy et al. 2012). Alternatively, the effects of factors different from successional age can be assessed through multivariate analysis (Austin 1977, Vítovcová et al. 2021).
Since the seminal works of Frederic E. Clements (1936) and Henry A. Gleason (1926), there has been a debate on the extent to which deterministic and stochastic processes influence the assembly of species throughout plant succession (Norden et al. 2015, Estrada-Villegas et al. 2020). Support for either position, or combining both, has emerged from different theoretical frameworks. Connell & Slatyer (1977) proposed three mechanisms of succession: (1) facilitation, when the arrival of new species modifies the environment, favoring the arrival of other species; (2) tolerance, when the colonization, permanence, and disappearance of species over time is a function of longevity and other life history attributes of the species; and (3) inhibition, when one colonizing species prevents or strongly limits the entry of other species. Pickett et al. (1987) proposed that causes determining modes of succession could be explored at three hierarchical levels. The upper level refers to three universal causes that trigger succession: the availability of open sites, species’ differential availability, and species’ differential performance. At a second level, these causes are broken down into ecological processes. At the third level, processes are analyzed in-depth to explore mechanisms. Modeling approaches have also been conducted to uncover possible mechanisms of species replacement driving succession based on deterministic characteristics (e.g., the ability to compete for light or soil resources, life history, or physiological attributes) of the species (e.g., Shugart & West 1980, Botkin 1981) or adopting a probabilistic approach (e.g., Horn 1975, Martínez-Ramos 1985). The neutral theory of Hubbell (2001), on the other hand, highlights stochastic, random events of migration, colonization, and local extinction of species as the main forces driving the course of succession.
Recently, using the conceptual framework of the so-called ecological filters to assess community assembly theory (HilleRisLambers et al. 2012) during succession (Chang & Turner 2019) has become common. According to this framework, the species participating during succession are filtered by abiotic and biotic factors from a regional species pool (Chang & HilleRisLambers 2016, Arroyo-Rodríguez et al. 2017). Abiotic filters are proposed to play a major role in early succession by selecting species with functional and life history attributes that allow them to cope with harsh environmental conditions, as documented in the tropical dry forest (Lebrija-Trejos et al. 2010; Pineda-García et al. 2016). Because the availability of light, space, water, or other resources may be high in open sites, few species with acquisitive traits and r-oriented life history strategies are expected to dominate early in succession (Chang & HilleRisLambers 2016 but see Ulrich et al. 2016). As succession advances, the harshness of abiotic conditions lessens, and resource availability decline. In these circumstances, biotic interactions (competition, herbivory, diseases) become the major filters, determining an increase in species diversity and turnover (Raevel et al. 2012, Chang & Turner 2019). In tropical wet forests, species dominating late succession have conservative traits (efficient in capturing and using limited resources) and K-oriented strategies (Lohbeck et al. 2014). These ideas have been tested using functional (e.g., Hernández-Vargas et al. 2019a, b, Raevel et al. 2012, Bhaskar et al. 2014, Csecserits et al. 2021), demographic (Lasky et al. 2014, Muscarella et al. 2017), and or phylogenetic approaches (Letcher et al. 2012, Purschke et al. 2013, Maza-Villalobos et al. 2020). Filters change in nature and magnitude depending on the vegetation type (Lohbeck et al. 2015, Poorter et al. 2021b) and as succession develops, differentially affecting distinct plant life cycle stages (Norden et al. 2012, Martínez-Ramos et al. 2021a).
Overall, it is likely that both determinism and stochasticity influence plant succession (e.g., Norden et al. 2015) and that the relative importance of deterministic and stochastic processes giving rise to succession depends on its biophysical context (Boukili & Chazdon 2017, Estrada-Villegas et al. 2020). Studies of plant succession have made notable progress in recent years, especially in tropical forest areas subject to agricultural activities (Poorter et al. 2016, Gei et al. 2018). These studies have shown a rapid recovery (< 20 years) of structural attributes (abundance, species richness) and functional traits (e.g., wood density, specific leaf area) and a slow recovery (> 120 years) of above-ground biomass and species composition (Poorter et al. 2021a). Therefore, secondary forests represent a great opportunity to conserve biodiversity, ecosystem functions, and services in landscapes modified by human activities (Chazdon 2014, Chazdon et al. 2016, Rozendaal et al. 2019).
In the absence of human intervention, abandoned fields normally undergo successional processes. However, the regeneration capacity of native vegetation is lost when the agricultural regime is severe, long-lasting, and extensive (Zermeño-Hernández et al. 2015), in which case succession may be deflected to vegetation dominated by weeds or exotic plants (Martínez-Ramos et al. 2016). Under these circumstances, restoration actions are required to recover the desired properties of the pre-disturbed vegetation (Arneth et al. 2021).
Short-term dynamics. Vegetation is renewed as plants’ birth, growth, and death unfold. The process of replacing plants that die with those germinated and growing anew is known as ‘natural regeneration’ (Martínez-Ramos 1994). This process is complex, as multiple changing abiotic factors (climate, microclimate, soil quality, topography) and biotic factors (pollination, frugivory, granivory, symbiosis with microorganisms, herbivory, parasites, pathogens) come into play, determining the production and dispersal of seeds, their germination and the survival and growth of seedlings, sprouts, and young plants towards adult life stages (Martínez-Ramos 1994, McCaughey & Tomback 2001, Vieira & Scariot 2006, Pausas et al. 2009).
Natural regeneration rates in plant populations and communities vary widely among regions of different latitudes. The pace is slow in cold or arid regions, where the freezing of water during the winter in the former (Forbes et al. 2005, Calama et al. 2017) or the lack of rain during several months in the latter (Copeland et al. 2021) limits plant regeneration. The rate is relatively fast in the warm-humid tropical regions, where few limitations for plant growth exist (Martínez-Ramos 1994). The rate of natural regeneration varies with soil nutrients and water availability within the same region, depending on edaphic and topographic factors (Minore & Laacke 1992, Rodriguez-Garcia et al. 2011).
In forests with a closed canopy in temperate or tropical regions, light energy in the understory is scarce, accounting for less than 10 % of the total solar radiation above the forest canopy (Chazdon & Pearcy 1991, Messier et al. 1998). Therefore, seedlings of trees whose foliage is exposed near the forest floor grow at slow rates, some in a dormant state (Bongers 1988Popma & , Kitajima 1996, Beaudet & Messier 1998). The opening of gaps in the canopy accelerates the natural regeneration process, increasing light availability in the undergrowth, leading to a gap regeneration process known as the ‘forest regeneration cycle’ (Martínez-Ramos 1994, Zhu et al. 2014).
The patch dynamics theory (Pickett & White 1985) or regeneration mosaic theory (Watt 1947) is linked to gap dynamics. Small openings (< 100 m2), produced by the fall of large tree branches, create ephemeral light pulses that stimulate the growth of seedlings and saplings for short periods (< 10 years; Yamamoto 2000, Martínez-Ramos et al. 1988a, b). Several tropical forest tree species grow towards the canopy using ephemeral gaps, but others can grow continuously in the shade (Brienen & Zuidema 2006, Brienen et al. 2010). The fall of one or several canopy trees opens large gaps (> 300 m2) where the regeneration of light-demanding, fast-growing, short-lived (< 50 years) trees occurs (Brokaw 1985, Sarukhán et al. 1985). Forests whose natural regeneration is accompanied by a regime of small clearings can complete regenerative cycles (or canopy renewal) of several hundred years, as was found in mangroves in the Dominican Republic (Sherman et al. 2000). In humid tropical forests, whose natural regeneration depends on small and large gaps, the regeneration cycle can be relatively fast (< 200 years; Martínez-Ramos et al. 1988a, Zhu et al. 2014). The environmental heterogeneity generated by gap dynamics, plus the biotic interactions between plants and animals, plants, and soil micro-organisms, as well as plants with each other, play a critical role in maintaining species diversity in different plant communities (Kimmerer & Young 1996, Nakashizuka 2001, Busing & Brokaw 2002, Wright 2002, Vandvik & Goldberg 2006, Martínez-Ramos et al. 2016, Mejía-Domínguez et al. 2021).
The natural regeneration of some plant species is facilitated by the presence of other species. Nurse plants are those whose presence generates micro-climatic conditions, soil properties, and or protection against natural enemies, increasing the survival, growth, or reproduction of other species (Callaway 2007, Filazzola & Lortie 2014). This phenomenon is most evident in the vegetation of arid zones, but it has been observed in moist ecosystems (e.g., Avendaño-Yáñez et al. 2014) and secondary tropical dry forest (e.g., Sánchez-Velásquez et al. 2004) as well. In desert ecosystems, natural regeneration proceeds through the interaction of plants with different growth forms and physiologies. Thus, succulent plants (cacti, agaves) with CAM-type metabolism become established and develop under shrubby plants with C3-type metabolism, establishing themselves in open sites (Castillo Landero & Valiente-Banuet 2010). When the succulent plants grow, the nurse plants may be favored by the water stored by the succulents (Montesinos-Navarro et al. 2019), but there is evidence that adult cacti can replace their nurses, possibly due to competitive effects (Valiente-Banuet et al. 1991).
Vegetation and conservation
We are facing a global process of replacement of natural vegetation, which took thousands or millions of years to emerge, with mono-crop systems, pastures for cattle ranching, plantations, and other large-scale, low species diversity agricultural land uses (Ellis & Ramankutty 2008). Many of these new ecosystems are maintained through human work and energy inputs (Hobbs et al. 2009, 2013). Vegetation has been a key concept in the definition and implementation of land management plans, including modern conservation actions since their origins in the 19th century. Although earlier cases of establishment of natural protected areas (NPA) date back several centuries, the establishment in 1872 of the Yellowstone National Park in the United States of America represents a landmark in nature conservation that was shortly followed in Australia (1879), and afterward in New Zealand, South Africa, South America, and elsewhere, mostly starting in the 1930s (Huntley et al. 2019, Sher & Primack 2019). In Mexico, the history of natural protected areas goes back to the last quarter of the 19th century when an area of fir (Abies religiosa (Kunth) Schltdl. & Cham.) forest nearby Mexico City was first devoted to the conservation of several water springs; eventually, this area became a national park in 1917 (Vargas Márquez 1997).
Ever since the beginning of conservation actions in NPA, the identification of target or flagship species (mostly vertebrates) has been a major driver of conservation policy and practice to attract public attention on national and international scales, with special actions on conservation of vegetation more diffusively defined (Meffe & Carroll 1994, Huntley et al. 2019). Yet national and regional biodiversity conservation management plans rely on vegetation and habitat maps (Mucina & Rutherford 2006, Keeler-Wolf 2007, Luxton et al. 2021), and the description and monitoring of vegetation units are now regarded as key elements to link conservation actions and impacts at four relevant levels of biodiversity: regional or landscape, community or ecosystem, population or species, and genetic (Noss 1990).
The adoption of the vegetation concept is akin to a holistic approach to conservation assessment and action. It should be noted that vegetation comprises complexities of the ecosystem that at the same time are amenable to being easily and routinely monitored (Dale & Beyeler 2001). According to these authors, vegetation includes a number of desirable attributes when used as an ecological indicator: (1) it may be easily measured, (2) it is sensitive and responds to environmental stresses in an integrative and predictable manner that allows anticipatory management actions, (3) it may have a known response to natural and human disturbance and changes over time, and (4) it has low variation in its response.
At least two vigorous developments of vegetation science-related research took place in the second half of the 20th century that contributed to a relative lack of interest in further development of vegetation studies by the ecological community. On the one hand, conceptual and methodological advances in population and community plant ecology have put an emphasis on disentangling the mechanisms behind the processes that we observe as vegetation patterns (e.g., Harper 1977, Solbrig 1980). Experiments and demographic studies conducted in the field, common gardens, or the laboratory have been preferred as the most promising approach to understand the workings of natural systems in the context of conservation biology. On the other hand, starting in the early 1970’s with the widespread availability of images from Landsat satellites on the Earth’s natural resources, the application of remote sensing technologies was accompanied by a relative neglect of field vegetation studies. Yet current challenges on monitoring threats on the vegetation cover of the planet driven by climate change and human land use call for a revival of vegetation science and soil classification linked to functional traits of dominant species groups as an effective tool for decision making (Luxton et al. 2021, Joswig et al. 2022).
In his insightful paper After description, Harper (1982) emphasized the need for detailed autoecological studies to understand the mechanisms that may be responsible for the broad patterns that we can identify when describing and classifying vegetation units (e.g., Shimwell 1971, Mueller-Dombois & Ellenberg 1974). An understanding of the workings of individuals and populations in response to their environment seems essential to approach the functioning of vegetation and the support it provides to ecosystem services. After their separate autoecological study, accumulating information makes it increasingly feasible to group plant species according to such relevant functional traits as seed germination, seedling and adult growth, and vegetative and reproductive phenology, among others (Fox 2018, Bórnez et al. 2020). The study of impacts of climate and soil alterations on these kinds of traits at the level of the whole plant community demands a merging of autoecology with vegetation science (Joswig et al. 2022).
In addition, unprecedented levels of spatial and spectral resolution will continue to develop within the realm of remote sensing technology. Major challenges will emerge to take full advantage of these capabilities in a revival of vegetation studies. Complementarity of the latest technological advances in both land-truthing and remote sensing with information on autoecological responses within the context of vegetation will provide novel insights at the spatial scale where most climate-change and development impacts will be detected and effected. Vegetation provides the scale at which a deeper understanding of evolutionary biology will be achieved and at which species will be identified to be protected (e.g., Luxton et al. 2021).
Conceptual and operative advances in conservation biology and practice, for its part, call for more holistic approaches to understand the complex relationships of nature and society. Complexity theory has emerged as the most recent promise of generating the amazing variety of life histories from the behavior of networks of simple entities related by numerous simple connections (Lewontin & Levins 2007). Yet decision-making in conservation planning and practice implies an explicit consideration of an integrative entity like “vegetation” to assess their effectiveness in the provision of environmental services to all stakeholders involved. Systematic Conservation Planning (SCP) is a current paradigm that aims at providing decision support for choices between alternative conservation actions (Margules & Pressey 2000). Currently, SCP is proposed as an iterative process aimed at the optimization of outcomes for biodiversity while minimizing societal costs; biodiversity conservation priorities are set to provide a quantitative interpretation of broader conservation goals for units of vegetation (McIntosh et al. 2017).
Setting up the heater: vegetation and climate change
Increasing temperatures due to climate change are currently recognized among the main threats to vegetation and its component plant species (García et al. 2014, Urban 2015, Pecl et al. 2017, Fox 2018). Effects of climate are not only expected to occur on the physiology of plants but also on metapopulation structure and connectivity due to interactions with increasing isolation caused by land clearing, increasing distances to diaspore sources, and the availability of suitable habitats for regeneration. The threat from climate change is thus becoming a local reality through the feedback with other impacts such as overexploitation, pollution, alien species invasion, and most notably, land-use change (Feddema et al. 2005, Newbold et al. 2015). Land transformation is a major global cause of ecosystem degradation (Mendelsohn 2019), particularly nowadays in the Global South (Winkler et al. 2021), and it has feedbacks that should be considered when simulating future climates (García et al. 2014, Pecl et al. 2017). Consistent global forest loss has been documented up to the first decade of the 21st century (e.g., Hansen et al. 2013). Major drivers of forest loss are net deforestation and land-use change in the tropics, intensive forestry activities in subtropical regions, and forestry and fires in boreal forests (updated to 2019 in https://data.globalforestwatch.org/documents/14228e6347c44f5691572169e9e107ad/explore).
Land cover-related impacts on global climate may occur through: (1) altering the rate of biogeochemical cycles and changes in the chemical composition of the atmosphere, or (2) altering physical parameters that influence energy absorption and disposition, notably on surface hydrology and gas exchange by different vegetation structure and roughness. Effects of changes in land cover have mainly a regional scope and can significantly alter regional climates (Bruijnzeel 2001, Feddema et al. 2005, Boone et al. 2011). Although land use has usually been considered a local environmental driver, its relevance has been proposed as probably the most significant cause of biodiversity loss on a global scale (Foley et al. 2005, Haddad et al. 2015, Newbold et al. 2015).
Global scenarios of ecosystem services and climate change need a better understanding of ecological mechanisms acting at the local level, so we may implement more effective actions to securing them while mitigating undesirable trends (Bennett et al. 2003). The revival of vegetation studies aimed to disentangle hidden structural and functional relationships with feedback from novel remote sensing technologies will enable more sophisticated planning and monitoring of natural resources, in addition to predicting not only global or broad-scale changes but at the more local needs of management programs. A clear example of this new reinvigorated frontier of vegetation science is the understanding of climate change and human land use impacts on such functional issues as plasticity in thermal tolerance, seed dispersal and germination, seedling growth, and phenology, among others, with its ensuing consequences on the biology and management of plant communities and populations (Fox 2018, Han et al. 2021, Fricke et al. 2022, Baskin & Baskin 2022).
Where do we go from here?
Ever since the first appearance of Homo sapiens more than 200,000 years ago (Hublin et al. 2017), human activities have impacted the Earth’s biodiversity and the legacies of these impacts on the present vegetation are evident (Sponsel 2013). Deforestation, land-use change, soil contamination, fires, exotic species invasion, defaunation, and global warming are all manifestations of human activities impacting the vegetation, especially in the last 150 years (Huntley & Baxter 2013).
The extraordinary thrust currently enjoyed by vegetation studies can be explained on several grounds. Among them, the technological development of computational tools for data analysis stands out, as it has facilitated access to, and the analysis of, an ever-increasing number of vegetation records, both for the plants occurring in the study communities and for the environmental factors affecting them. Such a great accumulation of information entails enormous challenges to understand vegetational patterns relative to its floristic composition, three-dimensional structure, plant diversity and functioning, and all processes potentially explaining these patterns.
In the future, a better integration of all the information that is being presently gathered in multiple vegetational systems should be based on generalized agreements on standardized methods that minimize differences between data sets that hinder their join analysis. This may be particularly critical for potentially conflicting information, for example, that relative to the degree of foliage persistence in the canopy, the canopy height itself, or the proper measurement of multi-stemmed woody plants. Another relevant topic for the advancement of our understanding of vegetation dynamics is the establishment of comparable permanent plots sufficiently spread out across vegetation variants and different spatial and temporal scales; in this way, we will be better able to represent different successional and non-successional dynamics and to gain insights into their potential drivers. In this context, a sensible goal may be the establishment of extensive plot networks with a minimum size of one hectare, in the case of forest communities, where the monitoring of all plant life forms, not only the woody ones, should be possible, including the ecological processes in which the plants are involved (e.g., González-M. et al. 2019, Steidinger et al. 2019).
Initiatives like this could strengthen through the integration of multidisciplinary research networks, both national and international, that will allow overcoming the limitations often encountered when pursuing the more complex goals frequently set up in regional projects, ultimately facilitating the understanding of vegetation processes across various scales (e.g., ForestPlots.net et al. 2021). Implementing such strategies will likely result in more robust vegetation classification systems, applicable over larger spatial scales (e.g., national or even continental). Attaining this objective would be crucial not only to gain an in-depth understanding of the ecology of all vegetation on the planet, but also to make significant progress on practical aspects, for example, the identification of high-priority areas for conservation, land use planning, the proper assessment of ecosystem services, or the monitoring of biodiversity changes caused by the climatic alteration that our planet is currently experiencing.











 nueva página del texto (beta)
nueva página del texto (beta)



