Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Abanico veterinario
versión On-line ISSN 2448-6132versión impresa ISSN 2007-428X
Abanico vet vol.13 Tepic ene./dic. 2023 Epub 25-Oct-2024
https://doi.org/10.21929/abavet2023.22
Original Article
Reproduction, age and growth of the carp Ctenopharyngodon idella (Valenciennes, 1844); of southeast Mexico
1Universidad Juárez Autónoma de Tabasco. División Académica Multidisciplinaria de los Ríos, México.
2El Colegio de la Frontera Sur, Unidad Villahermosa, Tabasco, México.
The purpose of the present study was to determine some aspects of the fishing biology, age and grown of Ctenopharyngodon idella during the 2018 annual cycle. One hundred specimens were collected from the Usumacinta River, Mexico. The gonosomatic (GSI), hepatosomatic (HSI) indexes, and condition factor (K) were determined. The age was estimated based on age rings counts in vertebrae and applying the von Bertalanffy growth model. Gonadal maturity stages were classified according to Nikolsky. The most frequent sizes were 60 and 70 cm TL. Males are smaller than females (Mann-Whitney, W = 2121.0 and 1094.5, p < 0.001). The length-weight relationship between females and males were statistically different (ANCOVA, F1.106 = 268.54, p < 0.001), and the general male-female proportion did not show differences (X211= 16.88, p = 0.111). The species presents two reproductive peaks (April and August). The relative fecundity is 78 (±28.63) oocytes/g. The studied population was constituted by ages between 1 and 9 years, being the age 4, 5 and 6 the most frequent. It is concluded that young specimens, probably due to high fishing pressure, compose the population structure of this species.
Keywords: Usumacinta River; fishery; cyprinid
El propósito del presente estudio fue determinar algunos aspectos de la biología pesquera, edad y crecimiento de Ctenopharyngodon idella durante el ciclo anual de 2018. Se recolectaron cien especímenes provenientes del río Usumacinta, México. Se determinaron los índices gonadosomático (IGS) y hepatosomático (IHS), así como el factor de condición (K) y se estimó la edad por medio la lectura de bandas de crecimiento en vértebras, aplicando el modelo de crecimiento de von Bertalanffy. Se clasificaron los estadios de madurez gonádica según Nikolsky. Las tallas más frecuentes fueron de 60 y 70 cm LT. Los machos son más pequeños que las hembras (Mann-Whitney, W = 2121.0 y 1094.5, p < 0.001). La relación longitud-peso entre hembra y machos fueron estadísticamente diferentes (ANCOVA, F1,106 = 268.54, p < 0.001), y la proporción general de sexos no mostró diferencias (X 211= 16.88, p = 0.111). La especie presenta dos picos reproductivos en abril y agosto. La fecundidad relativa es de 78 (±28.63) ovocitos/g. La población estudiada estuvo constituida por edades entre 1 y 9 años, siendo la edad 4, 5 y 6 las más frecuentes. Se concluye que la estructura de población de esta especie, está conformada por especímenes jóvenes, debido probablemente a la presión por pesca.
Palabras clave: río Usumacinta; pesquería; ciprínido
INTRODUCTION
The grass carp (Ctenopharyngodon idella) is one of the largest species of the Cyprinidae family. It is the only member of this genus and there are no known subspecies. Since the grass carp C. idella was introduced to Mexico at the end of the 19th century from China, it was widely distributed in northern and central Mexico, and it was later introduced in the rivers of southern Mexico. This species has outstanding qualities due to its high biological potential (high reproduction rates and adaptation to a variety of habitats and climates), resistance to handling and diseases. Ctenopharyngodon idella along with Cyprinus carpio and Carassius auratus are among the 25 exotic freshwater fish species for the central region of Mexico and together represent 12 % of dominant exotic species, being introduced to the country for aquaculture and weed control purposes (Baruah et al., 2014; Contreras-MacBeath et al., 2014; Silva et al., 2014; Ahmad et al., 2018).
In Mexico, it is cultivated in Aguascalientes, Chiapas, Coahuila, Durango, Guanajuato, Hidalgo, Jalisco, México, Michoacán, Puebla, Querétaro, San Luis Potosí, Sinaloa and Zacatecas states (INAPESCA, 2021). For the southeast of Mexico, and specifically for Tabasco and Chiapas states, this species has fishing importance (Mendoza-Carranza et al., 2013; Mendoza-Carranza et al., 2018), reaching in Tabasco a fishery production of 795 tons in live weight (CONAPESCA, 2021). Despite its fishery importance, grass carp represents a threat to the ecosystems where it occurs as its herbivory habits decrease habitat complexity by reducing plant structure (Amador-del ángel, 2014a; 2014b; Castillo-Domínguez, 2015).
Recent studies in C. idella are focused on cadmium toxicity effects, evaluation of hematological response after exposure to endosulfan, effects of copper sulfate on gill histopathology, structural changes in gills and musculature by exposure to chlorpyrifos and mercury. The effect of dietary alginate on growth performance and non-specific immunity of juvenile carp (Dahmardeh et al., 2012; Atabati et al., 2015; Bala, 2016; Kaur & Jindal 2016; Vajargah & Hedayati, 2017; Hu et al., 2021). On the other hand, studies on genetic diversity and phylogenetic relationships have been carried out in China, using SSR markers (Yu, 2014); as well as the variability of populations in China estimated using EST-SNP markers (Muhammad et al., 2022) and the analysis on the chemical composition and fatty acid profile of fillets (Hoseini, 2013). Likewise, evaluation of hematological and plasma indices in grass carp with reference to age, sex and hormone treatment (Ejraei et al., 2015). Other studies evaluate the weight-length ratio of the species fed with balanced food and comparative studies of the retina of the eye with other fish species (Bhosale & Bhilave, 2014; She et al., 2014) and pathologies caused by cestodes (Ahmad et al., 2018). As seen in Mexico, basic and applied research on this species is
very limited. The objective of this research is to determine some aspects of the reproductive biology, age and growth of the grass carp Ctenopharyngodon idella in the basin of the Usumacinta River, Tenosique, Tabasco, that will serve as base information for the implementation of ecological-fisheries strategies for a sustainable use and control of this introduced species.
MATERIAL AND METHODS
Study area and fish sampling
For this research, 100 specimens of C. idella were employed. These were collected during an annual cycle from January-December 2018 from commercial fisheries in the Usumacinta river basin, Tenosique, Tabasco. The catches of the specimens included the areas described by the fishermen as Crisóforo Chiñas (17°26'57.53 "N and 91°28'44.83 "W) and the locality of Chaculji (17°29'54.92 "N and 91°28'0.23 "W). The nets used for their capture were of the net tows (chinchorro) type with a mesh size of 4 cm, 80 m long and 3 m high.
Biometrics of the specimens
The total length (TL) and total width (TW) of each organism were obtained monthly, with a 100 cm ichthyomete with a precision of ±1.0 mm. Total weight (TW), gutted weight (GW), gonad weight (Wg) and liver weight (LW), were recorded with an OBI® 5000 g digital scale. All these with an accuracy of ±0.1 g, and a Torrey® 20 kg scale, with an accuracy of ±2 g. Sex determination in specimens was corroborated by direct observation of gonads during dissection (Rodríguez-Gutiérrez, 1992). Sex and maturity stages were determined based on the partial spawner maturity scales proposed by Nikolsky (1963), which comprises six stages: immature (I), inactive (II), maturing (III), mature (IV), spawning (V) and spent (VI).
Data analysis
Size frequency distribution was calculated, grouping organisms by 1 cm size class (Gulland and Rosenberg, 1992). An analysis of variance was used to test for statistical differences in overall means in TL after checking for normality and homogeneity of variances. The gonadosomatic index (GSI) was determined by the equation GSI= Wg / Wt x 100, where GSI= Gonadosomatic index, Wg= Gonad weight (g), Wt= Specimen weight (g) (Rodriguez-Gutierrez, 1992). The hepatosomatic index (HSI) was determined with the equation HSI= Wh / Wt x 100, where HSI = hepatosomatic index, LW = liver weight (g) and Wt = specimen weight (g) (Rodríguez-Gutiérrez, 1992). The physiological state or condition factor (K) of each specimen was obtained under the following equation: K = W / Lb x 100.
The length-weight relationship of C. idella was determined separately for both sexes by applying the equation TW= a TLb where TW= weight, TL= length, a= proportionality constant, b= the slope (growth coefficient).
Absolute fecundity was calculated as the relationship of maturing and mature oocytes in the ovary to the total fish weight. The estimate was developed by taking three subsamples of 0.3 g (±0.001) in three sections of the gonad (anterior, middle and posterior) and the equation Fa=n*G/g where G=total gonad weight, g = subsample weight, and n = average number of oocytes. Relative fecundity was estimated with the formula Fr=total oocytes/fish weight in g.
The average value of weight and length of males and females was compared using the Mann-Whitney (W) test for nonparametric data (Zar, 1999). A multiple correlation analysis and ANOVA was applied to the TL-TW regressions between sexes to identify possible differences between them (Sparre & Vanema, 1998). To determine possible differences in the monthly sex relationship, the Chi-square test (X2) was used (Underwood, 1996).
To establish the age of each specimen, the first thoracic vertebra was removed from each specimen, rinsed with water, dried and placed in polyethylene bags labeled with the collection data. Subsequently, they were roasted under the burning technique, for a better visibility of the growth rings. Clove oil was used for clarification. Growth rings were counted from photographs using a stereo microscope with a MOTICAM® 2.0 digital camera. For age determination, three readers were assigned to identify annual growth rings from digitized photographs, the first reader detected and counted each opaque zone as a growth ring, and the assigned age was replicated and verified by a second independent reader. The third independent reader established any discrepancies in age estimates between readers 1 and 2. The age frequencies of the specimens were recorded according to the readings established by the readers for each vertebra. The von Bertalanffy growth model Lt =L∞ (1-e-k(t-t0)) was applied to determine the age-length relationship, where: Lt = average total length at age t; L∞ = asymptotic total length; t0 = hypothetical length at age zero; k is the growth coefficient; and b is the slope of length. Growth parameters were considered for combined sexes using the Ford-Walford's linear method.
RESULTS
Immature specimens presented a TL 27.50 to 37.5 cm (56.18 ± 16.15 cm). Males presented a TL 61.53 to 77.54 cm (68.5 ± 3.78 cm) with the 60 cm class being the most frequent. In females the TL was 40.00 to 81.5 cm (72.1 ± 7.29), with the 70 cm class being the most frequent. Males were significantly smaller in length and weight (medians (M) = 68.5 cm TL 3439.0 g TW, than females with M =72.0 cm TL 3986.0 g TW (Mann-Whitney, W = 2121.0 and 1094.5, p < 0.001). The relationship for females was TW= 0.0457(TL)2.6617 and for males TW= 0.1182(TL)2.4290. For both sexes the model obtained was TW= 0.0526(TL)2.6244, and the percentage of variability of the data explained by the model (R2) was 88 % for females, 72 % for males and 85 % for both sexes. The observed value of b for the TL-TW equation for both sexes is less than three, indicating that the species presents a negative isometric growth type (t2 =1.00, p<0.005) (Figure 1). The length- weight relationship of female and males were significantly different (ANCOVA, F1.106 = 268.54, p < 0.001).
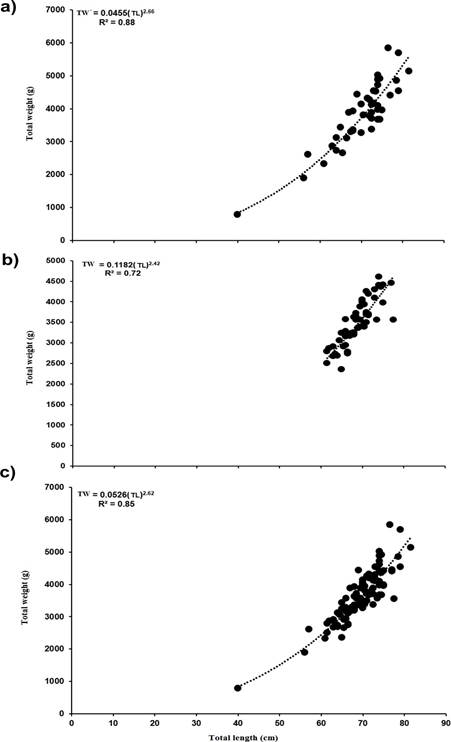
Figure 1 Length-weight relationship in females (a), males (b) and both sexes (c) of C. idella (N = 100) from the Usumacinta River, Tabasco, Mexico. TW = Total weight, TL = Total length, R2 = Coefficient of determination
Males accounted for 53 % of the organisms analyzed, while females accounted for 47 %, resulting in a male: female relationship of 1.1:1. The proportion of males was higher in February (88 %) and April (80 %), while females presented maximum values in May (89 %) and August (80 %). The relationship between males and females was statistically different in February (X211= 4.5, p = 0.034) and May (X211= 5.4, p = 0.020). The overall sex relationship showed no difference (X211= 16.88, p = 0.111). In October and November, it was not possible to capture specimens, due to the increase in river level.
The maximum GSI values in females occurred during April (15.52) and August (9.12). In January, June and December the GSI was less than 2.8. In males, the maximum GSI values were observed in July (2.17) and September (2.41), in the months of January, June and December the GSI was below 0.68 (Figure 2).
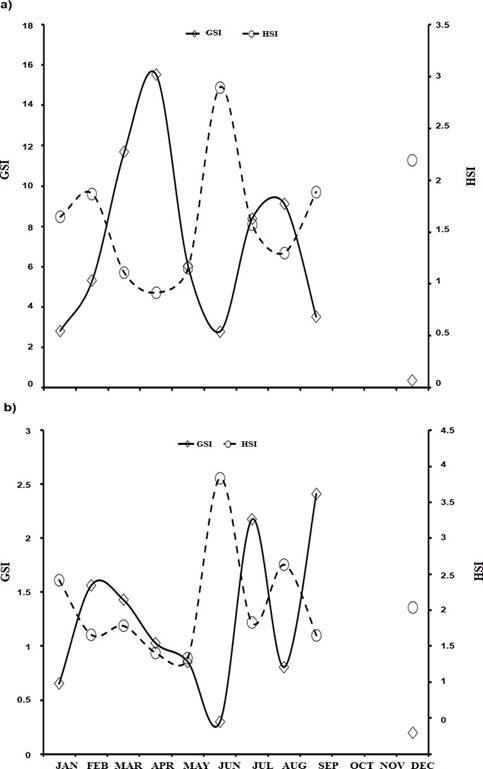
Figura 2 Monthly variation of the gonadosomatic index (GSI) and hepatosomatic index (HSI) in females (a) and males (b) of C. idella from the Usumacinta River, Tabasco, Mexico (N = 100)
The hepatosomatic index (HSI) in females showed its maximum values in June (2.91) and December (2.3) while the minimum was recorded in April (1.17). For males, the maximum values were recorded in June (3.18) and August (2.33) and the minimum in May (1.17). K values for both sexes had little variability throughout the year, showing a slight increase for females in March, June and September, with maxima in March for females (1.69) and December for males (1.59). The minimum values for females were 1.29 during April and 1.14 for males in August. The macroscopic description of the maturity stage of C. Idella gonads is described in Table 1 and Figure 3.
Table 1 Macroscopic description of gonadal maturity stages of C. idella from the Usumacinta River, Tabasco, Mexico (based on Nikolsky, 1963)
| Stage | Description |
|---|---|
| Immature (I) | Ovary small and elongated, few translucent, near the dorsal wall and below the swim bladder. Testes are pinkish filiform and poorly vascularized, with a location similar to that of the ovaries. They occupy approximately ½ half of the coelomic cavity. |
| Inactive (II) | Ovaries are enlarged pink, vascularized with a cylindrical appearance in the posterior part. The oocytes are not distinguishable macroscopically. They occupy approximately 1/2 to 1/3 of the coelomic cavity. Elongated testes with a slight increase in size, red coloration and increased vascularization. |
| Maturing (III) | Ovaries increased in size of uniform olive green color, dilated vascularization along the gonad, oocytes observed with the naked eye in the form of granules. They occupy 2/3 of the coelomic cavity. Testes markedly increased in size and soft consistency, the appearance of lobules on the periphery of the testis is appreciated. They are white in the posterior part and pinkish in the anterior part. |
| Mature (IV) | Ovaries of yellow color, with increased vascularization, occasionally with lobule formation along the gonad due to the increase in size of the oocytes. They occupy between 2/3 of the coelomic cavity. White colored testis, increased in size with amorphous aspects by lobules arranged lengthwise, indicating an increase in content. |
| Spawning (V) | Ovaries of partially flaccid with spherical oocytes, some oocytes transparent and hydrated prior to being expelled. They completely occupy the coelomic cavity and show slight vascularization. The testis is increased in size with amorphous lobules more marked than in the previous stage. The seminal fluid is expelled with simple pressure. |
| Worn (VI) | Very flaccid ovaries with distended membranes and hemorrhage in the anterior part. They occupy 1/3 of the coelomic cavity. Remaining oocytes with granular appearance are observed. Testis occupying the coelomic cavity similar to the ovaries. Reduced in size with flaccid appearance in the anterior part, slightly thickened middle part and posterior part of greater thinness, due to the expulsion of sperm fluid. |
Absolute fecundity in C. idella (n = 26) was in females weighing 2.318 - 5.845 g (X = 4.091g ± SD 771.34 g). This fecundity was 36.736 - 607.780 oocytes (X = 320.887 ± SD = 133.549.38). Relative fecundity was 14 - 125 oocytes/g (X = 78 oocytes/g ± SD=28.63 oocytes/g).
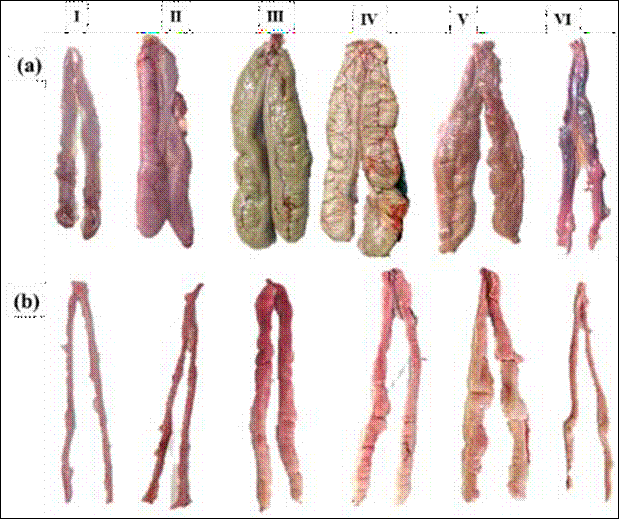
Figure 3 Images of gonad maturity stages in females (a) and males (b) of C. idella captured in the Usumacinta River, Tenosique, Tabasco. I = Immature, II = Inactive, III = Maturing, IV = Mature, V = Spawning and VI = Worn
The studied population of Ctenopharyngodon idella from the Usumacinta River consisted of eight age groups between 1, 3 and 9 years (Figure 4). For both sexes, ages 4, 5 and 6 were the most frequent (28 %, 25 % and 22 %, respectively). It is important to mention that no specimens belonging to age group 2 were obtained. The average error rate among the three readers was 8.26 % with a coefficient of variation of 8.45 %.
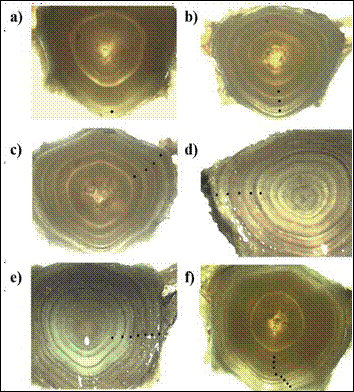
Figure 4 Images of vertebrae of C. idella showing the age of organisms captured in the Usumacinta River, Tabasco, Mexico. Black dots represent age: one-year (a), three years (b), four years (c), five years (d), seven years (e) and eight years (f)
The von Bertalanffy growth equation for both sexes was TL∞= 72.03(1-e0.46(t-0.553)), for females was TL∞= 74.26(1-e0.55(t-0.5568)) and for males was TL∞= 51.02 (1-e0.289(t-0.280)).
Water temperature ranged from 23°C in December to 30°C in May. Precipitation ranged from 1.9 mm in March to 22 mm in November. The Usumacinta River level reached its lowest value in April, at 11 m a.s.l., obtaining the greatest depth (19.11 m) in October. Rainfall was high throughout the second half of the year, beginning in June with 10.7 mm and ending in December with 14 mm.
DISCUSSION
This research is the first to be conducted on the reproductive biology of grass carp in the hydrological accounts of Mexico, so it was not possible to compare our data with previous studies, since most studies are focused on its fisheries importance (INAPESCA, 2021; Mendoza-Carranza et al., 2018). Despite this lack of studies related to biological-fishery data, it is observed that the TL (cm) and weight (g) recorded were similar to growth in captivity (Sherrat, 2020). The frequency in TL and weight recorded was lower than that reported for this same species in Western Europe, but were similar to those recorded in the fishery resources of the Usumacinta River in Mexico (Milardi et al., 2015; Mendoza- Carranza et al., 2018). The size structure of C. idella depends on the environment in which they live, mainly in terms of food availability, population density and ecological factors. Therefore, the recording of these lengths in this study may be conditioned by a migratory behavior of the species, as is the case of other species, such as Labeo rohita, Barbus graellsii, Chondrostoma miegii (Miñano et al., 2000; Mir et al., 2013).
Analysis of length-weight relationship for females and males indicates that these are statistically different, similar data to those recorded by Hailu, (2013) and Khalid & Naeem, (2017) in a tropical reservoir (Amerti: Ethiopia) and Southeastern Pakistan, respectively. The male: female ratio in the present study was similar, the records by Hailu, (2013) for common carp (Cyprinus carpio). However, in the present study there was difference in this ratio in the month of February that was favorable to males and on May was favorable to females. It is relating it to the reproductive indices for these months; it can be observed that males mature first than females, with the purpose of preparing to spawn in the month of April, where the highest gonadosomatic index was recorded.
The GSI in C. idella indicates an activity starting in February with a maximum peak in April and August in females, behavior that is related to the reproductive peaks of males during the annual cycle studied. These records are similar to those reported in the Nile River at a temperature of 20 °C. As well as with specimens from a reservoir in Sri Lanka, where reproductive peaks occur in the month of October. However, in the present study, no specimens were captured in this month due to the increase in the level of the Usumacinta River (Abd-Elhakim et al., 2019; Nathanael & Edirisinghe, 2021; Sheha et al., 2021). In carp, final maturation and spawning could occur within a 24-h period following environmental cues (water temperature, dissolved oxygen) and stimulating factors (specific climatic conditions, the presence of herbaceous vegetation, opposite sex and rainfall or flooding) for spawning to be present, and this likely accounts for increased GSI during the reproductive period. If these signals do not appear within a certain period, at the gonadal level, atresia or reabsorption of a portion of the vitellogenic oocytes occurs (Nathanael & Edirisinghe, 2021). On the other hand, C. idella has high reserves of essential fatty acids such as Ω-3 and Ω-6, which contributes to the reproductive success of the species (Bibi & Muhammad, 2021).
The hepatosomatic index for C. idella in the present study shows similarity to that recorded for the species in the Nile River of Egypt (Abd-Elhakim et al., 2019).
In the present study the length distributions were relatively wide, ranging from 55 to 110 cm, which, are within the age of first sexual maturity in males of 53 cm in length reported by Sheha et al., (2021) and those reported by Abd-Elhakim et al., (2019) of 62 cm for females and 53 cm for males.
The fecundity of the species in this study resulted similar to that reported by Maiztegui, (2015); Khalid & Naeem, (2017) and Jones et al., (2017) for C. idella and C. carpio, respectively.
One of the important environmental parameters for the growth and reproduction of grass carp is water temperature. In this research resembles those reported by other authors on the biological aspects of the species, mentioning tolerances from 0 to 33 °C. It, and that temperatures above 38 °C, that are lethal for adults, and the water temperature in which they are stimulated to sexual maturity is between 20 to 30 °C, but generally between 20 and 22 °C. These parameters are similar to those recorded for the species Cyprinus carpius (Laverde & Hernando, 2012, Tessema et al., 2020).
The most frequent ages of C. idella in the present study for both sexes were 4, 5 and 6 years, being these ages different from those reported in Eastern Europe (from 7 to 17 years) (Milardi et al., 2015), these differences may indicate the impact of the fishery to which the species is subjected in the Usumacinta River. In addition, they are within the age recorded in this same species in two reservoirs: Patterson and Bowman-Haley in North Dakota in the U.S. State, which ranged between 2 and 24 years through the study with fin rays and between 2 and 27 years with dorsal fin spines (Weber et al., 2011). Other species studied in the same family present this variability; such is the case of the species Labeo rohita where the highest age recorded was 8 years with a TL of 86.22 to 90. 45 cm; also in the species Barbus graellsii and Chondrostoma miegii recording specimens of 10 and 8 years of age, and of Gobio lozanoi with ages of 7 and 8 years; Luciobarbus callensis of 4 and 7 years. To some extent, these variations are the product of the plasticity of the species to adapt to different environments (Miñano et al., 2000; Amat-Trigo et al., 2013; Mir et al., 2013; Mimeche et al., 2013).
In this study, the adaptability of Ctenopharyngodon idella to the environmental and ecological conditions of the Usumacinta River in southeastern Mexico is confirmed. However, being an exotic species, it is among the most important, least controlled and irreversible impacts that occur in ecosystems and that affect their biodiversity in a very important way; as well as, they can strongly modify the hydrology, the biogeochemical cycle and the biotic composition of invaded ecosystems (Strayer, 2010; Traveset, 2015). On the other hand, the large volumes with which species are caught have positively impacted the economy of fishermen, since, although they do not have a high commercial value, they represent a good income for their families. Likewise, these species have modified the dietary patterns of the surrounding communities, a source of affordable animal protein for these communities due to the economic value at which they are marketed (Mendoza-Carranza et al., 2018).
CONCLUSION
The biological-fishing aspects demonstrate the adaptive capacity of the species in southeastern Mexico, specifically in the Usumacinta River basin. It is a species with two maximum peaks of sexual maturity, in July and September for males, and in April and August for females. The most frequently captured ages of C. idella for both sexes were 4, 5 and 6 years old. This indicates that, according to the data obtained in this research, fishing exploitation is mainly of young organisms. It is important to continue with these studies to provide biological, ecological and commercial information in different geographic and temporal areas with the objective of establishing a fishing management of this species that is being subjected to strong fishing pressure.
LITERATURA CITADA
ABD-ELHAKIM E, El-Gamal Samah TD, Mohamed AS. 2019. The reproductive biology of grass carp (Ctenopharyngodon Idella) collected from floating cages in River Nile, Egypt. East African Scholars Journal of Biotechnology and Genetics. 1(6): 149-157. ISSN: 26637286. https://www.google.com/search?q=doi%3A+10.36349%2Feasjbg.2019.v01i06.004&client=opera&hs=ZGT&sxsrf=ALiCzsZlE3EyVyOBWMqY4FjaCwFyxAM5LA%3A1662514312197&ei=iPQXY9rgC-bPkPIPovu-oAU&oq=DOI%3A+10.36349%2Feasjbg.2019.v01i06.004&gs_lcp=Cgdnd3Mtd2l6EAEYADIECCMQJ0oECEEYAUoECEYYAFDVG1jwRWDfVmgDcAB4AIAB1gGIAb4IkgEFMC42LjGYAQCgAQHAAQE&sclient=gws-wiz [ Links ]
AHMAD F, Fazili KM, Sofi OM, Sheikh BA. Sofi TA. 2018. Distribution and pathology caused by Bothriocephalus acheilognathi, Yamaguti 1934 (Cestoda: Bothriocephalidae): a review. Revista Veterinaria. 29: 2:142-149. ISSN 1669-6840. https://core.ac.uk/download/pdf/230828318.pdf [ Links ]
AMADOR-DEL ÁNGEL LE, Wakida-Kusunoki AT. 2014a. Peces invasores en el sureste de México, en R. Mendoza y P. Koleff (coords.), Especies acuáticas invasoras en México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, México, pp. 425-433. ISBN 978-607-8328-04-8.https://www.researchgate.net/publication/259868683_Peces_invasores_en_el_Sureste_ de_Mexico#fullTextFileContent [ Links ]
AMADOR-DEL-ÁNGEL LE, Wakida-Kusunoki AT. 2014b. Especies acuáticas exóticas e invasoras del estado de Tabasco, México. In: Low Pfeng AM, Quijón PA, Peters Recagno EM (eds). Especies invasoras acuáticas: casos de estudio en ecosistemas de México. Ciudad de México, México. Pp 177-198. ISBN: 978-1-304-90189-7. https://www.researchgate.net/publication/268447867_Especies_acuaticas_exoticas_e_i nvasoras_del_estado_de_Tabasco_Mexico#fullTextFileContent [ Links ]
AMAT-TRIGO F, Oliva-Paterna FJ, Verdiell-Cubedo D, Ruiz-Navarro A, Torralva M. 2013. Edad y crecimiento de Gobio lozanoi Doadrio & Madeira, 2004 (Cypriniformes: Cyprinidae) en sectores fluviales de la cuenca del río Segura (SE Península Ibérica). Anales de Biología 35: 109-121. ISSN 1989-2128. https://revistas.um.es/analesbio/article/view/325491/227331 [ Links ]
ATABATI A, Keykhosravi A, Askari-Hesni M, Vatandoost JM. 2015. Effects of Copper Sulfate on gill histopathology of grass carp (Ctenopharyngodon idella). Iran Journal Ichthyology. 2(1): 35-42. ISSN 2383-0964.http://www.ijichthyol.org/index.php/iji/article/view/13 [ Links ]
BALA SR. 2016. Evaluation of haematological response of grass carp (Ctenopharyngodon idella) after exposure to endosulfan. International Journal of Fisheries and Aquatic Research 1: 01-04. ISSN: 2456-7248. https://www.fishjournals.com/assets/archives/2016/vol1issue1/1-1-16-829.pdf [ Links ]
BARUAH UK, Rabha HP, Mazumdar M. 2014. Biocontrol of weed in summer rice through grass carp (Ctenopharyngodon idella). Scholars Journal of Agriculture and Veterinary Sciences. 1(3):143-148. ISSN 23481854.https://www.researchgate.net/publication/306059235_Biocontrol_of_weed_in_summer_r ice_through_grass_carp_Ctenopharyngodon_idella#fullTextFileContent [ Links ]
BHOSALE SV, Bhilave MP. 2014. The length weight relationship of Ctenopharyngodon idella fed with formulated feed. International Journal of Fisheries and Aquatic Studies. 2(2): 227-231. ISSN: 2347-5129.https://www.fisheriesjournal.com/archives/2014/vol2issue2/PartD/39.pdf [ Links ]
BIBI C, Muhammad A. 2021. Size dependent variation in cholesterol and fatty acids profile in different tissues of freshwater cyprinid Ctenopharyngodon idella. Commonwealth Journal of Academic Research. 2: 1-14. http://doi.org/10.5281/zenodo.4603244 [ Links ]
CASTILLO-DOMÍNGUEZ A, Melgar Valdes CE, Barba Macías E, Rodiles-Hernández R,A. de J. Navarrete, M. A. Perera García, C. A. Cuenca Soria y R. E. Hernández Gómez. 2015. Composición y diversidad de peces del río San Pedro, Balancán, Tabasco, México. Hidrobiológica. 25 (2): 285-292. ISSN 2448-7333. https://hidrobiologica.izt.uam.mx/index.php/revHidro/article/view/486/84 [ Links ]
CONAPESCA. 2021. Anuario estadístico de acuacultura y pesca de la comisión nacional de acuacultura y pesca. México. https://nube.conapesca.gob.mx/sites/cona/dgppe/2021/ANUARIO_ESTADISTICO_DE_ ACUACULTURA_Y_PESCA_2021.pdf [ Links ]
CONTRERAS MT, Gaspar-Dillanes, MT. Huidobro-Campos L, Mejía-Mojica H. 2014. Peces invasores en el centro de México, En: Especies acuáticas invasoras en México Mendoza R. y Koleff P. (Ed.). Pp. 413-424. ISBN 978-607-8328-04-8.https://www.researchgate.net/publication/263273578_Peces_invasores_en_el_Centro_de_Mexi co#fullTextFileContent [ Links ]
DAHMARDEH BR, Sahebi S, Sepehrikia S. 2012. Mercury contamination in muscle and scales of Grass Carp (Ctenopharyngodon idella) and Silver Carp (Hypophthalmichthys molitrix) of Zabol Chahnimeh Reservoirs (Iran). World Applied Sciences Journal. 20 (4): 565-569. ISSN 1818-4952.https://www.researchgate.net/publication/288442079_Mercury_contamination_in_muscl e_and_scales_of_Grass_Carp_Ctenopharyngodon_idella_and_Silver_Carp_Hypophthal michthys_molitrix_of_Zabol_Chahnimeh_reservoirs_Iran [ Links ]
EJRAEI F, Ghiasi M, Khara H. 2015. Evaluation of hematological and plasma indices in grass carp, Ctenopharyngodon idella, with reference to age, sex, and hormonal treatment.Archives of Polish Fisheries. 23: 163-170. ISSN 2083-6139 https://doi.org/10.1515/aopf-2015-0019 [ Links ]
GULLAND JA, Rosenberg AA. 1992. A review of length-based approaches to assessing fish stocks. FAO Fisheries Technical Paper. No. 323. Rome. ISBN:92-5-103121-5. [ Links ]
HAILU M. 2013. Reproductive aspects of common carp (Cyprinus carpio L, 1758) in a tropical reservoir (Amerti: Ethiopia). Journal of Ecology and the Natural Environment. 5(9): 260-264. ISSN 2006-9847. https://academicjournals.org/journal/JENE/article-full-text-pdf/D63E1AA11935 [ Links ]
HOSEINI M, Baboli MJ, Sary AA. 2013. Chemical composition and fatty acids profile of farmed Big head carp (Hypophthalmichthys nobilis) and Grass carp (Ctenopharyngodon idella) filet. AACL Bioflux. 6(3):202-210. ISSN 18449166. https://www.researchgate.net/publication/265726058_Chemical_composition_and_fatty_acids_profile_of_farmed_Big_head_carp_Hypophthalmichthys_nobilis_and_Grass_carp_Ctenopharyngodon_idella_filet [ Links ]
HU J, Zhang J, Wu S. 2021. The growth performance and non‑specific immunity of juvenile grass carp (Ctenopharyngodon idella) affected by dietary alginate oligosaccharide. 3 Biotech.11:46. ISSN 21905738. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7801570/ [ Links ]
INAPESCA. 2021. Instituto Nacional de Pesca. Secretaria de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. Diario Oficial de la Federación. Pp. 1-80. https://www.gob.mx/cms/uploads/attachment/file/630145/DOF_-_Diario_Oficial_de_la_Federacin_CNA.pdf [ Links ]
JONES LA, Mandrak, NE, Cudmore B. 2017. Updated (2003-2015) Biological Synopsis of Grass Carp (Ctenopharyngodon idella). DFO. Can. Canadian Science Advisory Secretariat Research Document. 2016/102. iv + 63 p. https://waves-vagues.dfo-mpo.gc.ca/library-bibliotheque/40599711.pdf [ Links ]
KAUR M, Jindal R. 2016. Sem study of ultrastructural changes in branchial architecture of Ctenopharyngodon Idella (Cuvier & Valenciennes) exposed to chlorpyrifos. Archives of Biological Sciences. 68(2):393-398. ISSN 26833867. https://www.serbiosoc.org.rs/arch/index.php/abs/article/view/781 [ Links ]
KHALID M, Naeem M. 2017. Morphometric relationship of length-weight and length length of farmed Ctenopharyngodon idella from Muzaffar Garh, Southern Punjab, Pakistan. Punjab University Journal of Zoology. 32(1): 57-64. ISSN 23138556. http://pu.edu.pk/images/journal/zology/PDF-FILES/9_V32_1_2017.PDF [ Links ]
LAVERDE BA, Hernando LPR. 2012. Growth of Common Carp (Cyprinus Carpio Carpio, Linneaeus, 1758) in Floating Cages in a Reservoir of Cajicá (Cundinamarca), Colombia. Revista Faculta de Ciencias Basicas. 8(2): 268-289. ISSN 19004699. https://revistas.unimilitar.edu.co/index.php/rfcb/article/view/2040/1677 [ Links ]
MAIZTEGUI T. 2016. Ecología poblacional de Cyprinus carpio (TELEOSTEI) en los Humedales de Ajó, Buenos Aires (Doctoral dissertation, Facultad de Ciencias Naturales y Museo). http://sedici.unlp.edu.ar/bitstream/handle/10915/51880/Documento_completo.pdf-PDFA.pdf?sequence=4&isAllowed=y [ Links ]
MENDOZA-CARRANZA M, Arévalo-Frías W, Inda-Díaz E. 2013. Common pool resources dilemmas in tropical inland small-scale fisheries. Ocean & Coastal Management. 82:119-126. ISSN: 0964-5691.https://www.sciencedirect.com/science/article/abs/pii/S0964569113001580?via%3Dihub [ Links ]
MENDOZA-CARRANZA M, Arévalo-Frías W, Espinoza-Tenorio A, Hernández-Lazo C, Álvarez-Merino AM, Rodiles-Hernández R. 2018. La importancia y diversidad de los recursos pesqueros del río Usumacinta, México. Revista Mexicana de Biodiversidad. 89: S131-S146. ISSN 2007-8706.https://revista.ib.unam.mx/index.php/bio/article/view/2182 [ Links ]
MILARDI M, Lanzoni M, Kiljunen M, Torniainen J, Castaldelli G. 2015. Natural recruitment contributes to high densities of grass carp Ctenopharyngodon idella (Valenciennes, 1844) in Western Europe. Aquatic Invasions. 10: 439-448. ISSN 1818-5487. http://www.aquaticinvasions.net/2015/AI_2015_Milardi_etal.pdf [ Links ]
MIMECHE F, Mohamed B, Ruiz-Navarro A, Oliva-Paterna FJ. 2013. The population structure, age and growth of Luciobarbus callensis (Cyprinidae) in a man-made lake in the Maghreb (NE Algeria). Limnetica. 32 (2): 391-404. ISSN 02138409. https://www.limnetica.com/documentos/limnetica/limnetica-32-2-p-391.pdf [ Links ]
MIÑANO PA, Oliva-Paterna FJ, Fernández-Delgado C, Torralva M. 2000. Edad y crecimiento de Barbus graellsii Steindachner, 1866 y Chondrostoma miegii, Steindachner, 1866 (Pisces, Cyprinidae) en el río Cinca (Cuenca Hidrográfica del Ebro, NE España). Miscel lania Zoologica. 23.2: 9-19. ISSN 02116529. https://raco.cat/index.php/Mzoologica/article/view/90055 [ Links ]
MIR JI, Sarkar, UK, Gusain OP, Dwivedi AK, Joukrushna J. 2013. Age and growth in the Indian major carp Labeo rohita (Cypriniformes: Cyprinidae) from tropical rivers of Ganga basin, India. Revista de Biología Tropical. 61: 1955-66. ISSN 0034-774. https://www.scielo.sa.cr/pdf/rbt/v61n4/a30v61n4.pdf [ Links ]
MUHAMMAD NKB, Tahir N, Aasma N, Aneela K, Muhammad B, Nabeela T, Nadia N, Tasleem K. 2022. Investigation of genetic diversity and phylogenetic relationship of Ctenopharyngodon idella from different regions of Punjab by using SSR Markers. Pure and Applied Biology. 11(1): 209-216. ISSN 23042478. https://thepab.org/index.php/journal/article/view/2303/1513 [ Links ]
NATHANAEL S, Edirisinghe U. 2021. Abundance and aspects of the reproductive biology of Common Carp Cyprinus carpio in an upland reservoir in Sri Lanka. Asian Fisheries Science. 14:343-351. ISSN 0116-6514. https://doi.org/10.33997/j.afs.2001.14.3.011 [ Links ]
NIKOLSKY GV. 1963. The ecology of Fishes. Academic, London and New York, USA. ISBN 0125197500, 9780125197502. [ Links ]
RODRÍGUEZ-GUTIÉRREZ M. 1992. Técnicas de evaluación cuantitativa de la madurez gonádica en peces. AGT, México, D.F., México. ISBN 9789788406549. [ Links ]
SHERRAT R. 2020. La Carpa Herbívora - Importante especie de Cultivo. International Aquafeed.https://aquafeed.co/entrada/la-carpa-herbivora-importante-especie-de-cultivo-22032#:~:text=La%20carpa%20herb%C3%ADvora%20(Ctenopharyngodon%20idella,por%20a%C3%B1o%20a%20nivel%20mundial [ Links ]
SHE Q, An Z, Xia C, Kong Y, Chen E. 2014. Study on comparative histology of retinas in Ctenopharyngodon idella, Cynops orientalis, Bufo gargarizans, Gekko japonicus and Columba livia. International Journal of Morphology. 32(3):918-922. ISSN 0717-9502. https://www.scielo.cl/pdf/ijmorphol/v32n3/art28.pdf [ Links ]
SHEHA AM, Darwish, TS, El-Gamal E, Barakat, RAO. 2021. Histological studies on the testicular cycle of grass carp, Ctenopharyngodon idella in related to gonadotrophic cells activity of the pituitary gland. Egyptian Journal of Aquatic Biology and Fisheries. 25: 141-160. ISSN 2536-9814.https://ejabf.journals.ekb.eg/article_145789_d19b8ae9892493e654da3f16b34613a3.pdf [ Links ]
SILVA AF, Cruz C, Pitelli RLCM, Pitelli RA. 2014. Use of Grass Carp (Ctenopharyngodon idella) as a Biological Control Agent for Submerged Aquatic Macrophytes. Planta Daninha. 32(4):765-773. ISSN 18069681.https://www.scielo.br/j/pd/a/b3PRrtSr5n9dz65Lhj7yJsg/?lang=en [ Links ]
SPARRE P, Venema SC. 1998. Introducción a la evaluación de recursos pesqueros tropicales, Parte 1. Manual. FAO, Santiago de Chile, Chile. ISBN: 9253039965. https://www.fao.org/3/w5449s/w5449s.pdf [ Links ]
STRAYER DL. 2010. Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshwater Biology. 55: 152-174. ISSN 1365-2427. https://doi.org/10.1111/j.1365-2427.2009.02380.x [ Links ]
TESSEMA A, Getahun A, Mengistou S, Fetahi T, Dejen E. 2020. Reproductive biology of common carp (Cyprinus carpio Linnaeus, 1758) in Lake Hayq, Ethiopia Fisheries and Aquatic Sciences. 23:16. ISSN 22341757. https://fas.biomedcentral.com/articles/10.1186/s41240-020-00162-x [ Links ]
TRAVESET A. 2015. Impacto de las especies exóticas sobre las comunidades mediado por interacciones mutualistas. Ecosistemas. 24(1): 67-75. ISSN 16972473. https://www.redalyc.org/pdf/540/54038707011.pdf [ Links ]
UNDERWOOD AJ. 1996. Experiments in ecology. Their logical design and interpretation using analysis of variance. Cambridge University, Cambridge, United Kingdom. ISSN 0521553296. https://www.cambridge.org/core/books/experiments-in-ecology/DCF3663D5E7C9923D19B5ECE88167780 [ Links ]
VAJARGAH MF, Hedayati A. 2017. Toxicity effects of cadmium In Grass Carp (Ctenopharyngodon Idella) and Big Head Carp (Hypophthalmichthys Nobilis) transylv. Transylvanian Review of Systematical and Ecological Research. 19.1 (43-48). https://intapi.sciendo.com/pdf/10.1515/trser-2017-0004 [ Links ]
WEBER MJ, Brown ML. 2011. Comparison of common carp (Cyprinus carpio) age estimates derived from dorsal fin spines and pectoral fin rays. Journal of Freshwater Ecology. 26:195-202 ISSN 2156-6941. https://www.tandfonline.com/doi/full/10.1080/02705060.2011.554218 [ Links ]
YU L, Bai J, Cao T, Fan J, Quan Y, Ma D, Ye X. 2014. Genetic variability and relationships among six grass carp Ctenopharyngodon idella populations in China estimated using EST-SNP Markers. Fisheries Science. 80:475-481. ISSN 14442906. https://link.springer.com/article/10.1007/s12562-014-0709-y [ Links ]
ZAR JH. 1999. Biostatistical analysis. 3th Edition. Prentice-Hall, Upper Seddle River New Jersey, USA. ISBN 013081542-X. [ Links ]
Received: September 10, 2022; Accepted: September 20, 2023; Published: October 08, 2023











 texto en
texto en 



