Study contribution
Shrimp farming is important in generating products of high nutritional value and economic value in different countries. This industry is constantly growing in different topics such as physiology, nutrition, adaptability, immune system, stress, and resistance to pathogens, etc. However, information regarding the immunological changes that shrimp undergo when exposed to immunostimulants is poorly understood. This study focuses on the use of C. maris, G. candidum, and Curtobacterium sp. as activators of the immune system of L. vannamei and evaluates their immune response (hemocytes, total proteins, and expression of genes) to this stimulus. The results showed that marine microorganisms can stimulate protein content in circulating hemocytes, antioxidant activity, and gene expression. This information can help shrimp farmers have a better control of pathogens and diseases of diseases that affect production.
Introduction
Pacific white shrimp (Litopenaeus vannamei) is one of the most cultivated crustacean species worldwide, with a global production of 1 237 016 t1 in 2022 and of 270 807 t in 2021 in Mexico. Furthermore, it is an important source of income.2 Due to the continuous demand for this product, shrimp farming has become an economically important activity, which is not immune among others, to nutritional, stress, environment, health, problems which are strongly associated with different factors that affect shrimp growth.3,4 Such problems in shrimp aquaculture favor the development of strategies to improve the health and growth of the organisms, the health through an early detection of diseases to reduce losses in production, and the reduction of environmental impact.1,5
The problems generated by pathogenic agents in shrimp (bacteria and viruses) have increased research on the immune system of these organisms using studies at cellular, protein, proteomic, and gene expression levels among others.6-8 An alternative for disease control is to activate or strengthen the shrimp immune system through the use of microorganisms, by-products, or cellular fractions thereof that improve resistance to pathogens.5 Some marine microorganisms contribute to the prevention of health problems that affect shrimp by having an excellent nutritional level, in addition to functional properties such as probiotics and/or immunostimulants, adhesion to the intestinal mucosa, antagonism against pathogens, and removal of organic matter.9-11
Different marine microorganisms are sources of 𝛽-glucans, lipopolysaccharides, and nucleotides that are used as immunostimulants in L. vannamei and provide resistance against bacterial and viral pathogens.9,12 They also generate the activation of genes of different immune factors such as antimicrobial peptide, anti-lipopolysaccharide factor, penaeidin, prophenoloxidase (ProPO), and SOD.13 Strains of microorganisms such as Candida maris, Geotrichum candidum, and Curtobacterium sp. have been used as a prophylactic measure against shrimp pathogens.14-16
Ochoa17 and Ochoa et al.18 reported an important response in cultured L. vannamei hemocytes exposed to G. candidum. This microorganism has been used as a probiotic agent in fish, where an increase in the immunological and productive response was found.19,20 This research aimed to evaluate the cellular immune response, protein content, and gene expression in juvenile Pacific shrimp (L. vannamei) exposed to C. maris, G. candidum, and Curtobacterium sp. isolated from the marine environment.
Materials and methods
Ethical statement
All animal experimental protocols were carefully revised and approved by an internal council at Centro de Investigaciones Biológicas del Noroeste or CIBNOR (Ethics Committee: CICUAL-CIBNOR, Spanish acronym, Institutional approval number CIBNOR-CEI-2023-04) and complied with the guidelines established by the European Union Council (2010/63/EU) and the Mexican Government (NOM-062-ZOO- 1999) for the cultivation, care, and use of experimental animals.
Microorganisms strain
The experimental strains used were Candida maris (A1), Geotrichum candidum (A3), and Curtobacterium sp. (S13), isolated from the sediment in the mangrove area of La Paz Bay, Baja California Sur, Mexico (24°14̛’59.80” N, 110°18̛’47.12” W) and characterized in vitro and in vivo at the Humid Laboratory for Crustacean Culture and Marine Immunogenomics Laboratory at the CIBNOR, La Paz, Baja California Sur, Mexico.21 The experimental strains were cultivated in YPD broth (Peptone-Dextrose for yeast) + 2.5 % NaCl with 50 µg/mL of chloramphenicol at 30 °C for 24 h. The non-hemolytic capacity of the strains (gamma hemolysis) was previously determined by the hemolysis test on blood agar plates + 2.5 % NaCl + 10 % rabbit plasma, using the puncture procedure (6 mm approx.) and incubation at 35 °C for 24 h.22 The controlled dose was generated by culturing the strains in YPD agar + 2.5 % NaCl at 30 °C for 24 h. The obtained colonies were suspended in a sterile 2.5 % NaCI solution until they reached an absorbance of 1 at 540 nm (1 × 106 CFU/mL of final dose).23
Bioassay
The bioassay was performed using juvenile Pacific white shrimp, Litopenaeus vannamei (14.25 ± 1.5 g), in the CIBNOR laboratory for acclimatization and culture of aquatic organisms. The bioassay was performed in fiberglass tanks with 30 L of filtered seawater (1 µL) at 28 ± 1 °C, 37 ± 2 ups, and continuous aeration (dissolved oxygen of 5.02 ± 0.5 mg/mL). Shrimp were randomized in triplicate at a density of 15 organisms/tank and exposed to five treatments for 216 h: (1) shrimp fed a commercial shrimp diet (PIASA, 35 % protein) as a negative control (TCN); (2) shrimp exposed to laminarin (β-1,3 glucan; SIGMA) as a positive control (TCP); (3) shrimp exposed to Candida maris (TCM); (4) shrimp exposed to Geotrichum candidum (TGC); and (5) shrimp exposed to Curtobacterium sp. (TCS). The replicates of each treatment were accommodated in three blocks of five tanks, where each block had a treatment that was also randomly distributed.
The microorganisms were applied to the corresponding treatments (TCM, TGC, TCS) by immersion at a dose of 1 × 106 CFU/mL to the respective experimental tanks.23 The TCP dose was based on the manuscript of Campa-Córdova et al.24 with the use of laminarin (Laminaria digitata, Sigma, L-9634) at a final concentration of 0.5 mg/mL. The TCN treatment contained only commercial feed. The dose of each treatment was applied at 0, 48, 96, 144, and 192 h. During the bioassay, daily maintenance was performed early in the morning and included the removal of molts and dead shrimp and siphoning of residual food and fecal material from the tanks. In addition, a daily exchange of 15 % of the total volume of seawater was conducted. The shrimp were fed ad libitum with the commercial diet (PIASA, 35 % protein) twice a day (09:00 and 15:00 h) at 1.9 % of the total biomass.
Sampling
Hemolymph from three rando (mL) and selected shrimp, one from each replicate, was collected at 24, 48, 72, and 216 h for evaluation of total hemocyte count (THC), total protein (TP), and gene expression analysis. Selected shrimps were euthanized by placing them in filtered seawater at 8 °C for 30 min, and 2 g of muscle was dissected from each shrimp. These tissues were stored at -80 °C to determine the superoxide dismutase (SOD) enzyme activity.
For hemolymph sampling, an isotonic shrimp solution with EDTA, an anticoagulant, SIC-EDTA (450 mM NaCl, 10 mM KCl, 10 mM EDTA-Na2, 10 mM HEPES, pH 7.3, and 850 mOsm/kg) was used to obtain the hemolymph sample.24 Hemolymph was obtained from the ventral hemolymphatic sac located at the base of the pleopod of the first abdominal segment near the genital pore, with a 5 (mL) syringe (22 × 32, 23 G) + 500 µL of SIC-EDTA at 4 °C (depending on the volume of hemolymph collected, the dilution factor was calculated).
The extracted hemolymph (1 mL approx.) was deposited in 1.5 mL microcentrifuge tubes (removing the needle so as not to break the cells) and cooled on an ice bed.(24, 25) 50 µL of collected hemolymph was used for total hemocyte count and protein analysis. The rest of the collected sample was used for gene expression and centrifuged at 3000 g for 3 min at 4 °C. The supernatant was removed and 500 µL of an ARNlater solution (InvitrogenTM, catalog number AM7021) was applied to the cell package6 for preservation at 4 °C for 24 h and subsequent storage at -80 °C, according to the manufacturer’s instructions.
Total hemocyte count
Total hemocyte count (THC) was performed as described by Campa-Córdova et al.24 and Pacheco et al.25, where 200 mL of 10 % formaldehyde was added to 50 µL of hemolymph. The hemolymph sample was evaluated in a Neubauer chamber to determine the number of hemocytes using a 10× binocular optical microscope (CX-21). The number of hemocytes was evaluated in cells per milliliter according to the following formula:
Protein quantification
The evaluation of TP (mg/mL) in the hemocytes and muscle was performed using a Bradford modification,26 analyzing the sample in a microplate at 595 nm. A final homogeneous hemocyte concentration was calculated for protein determination (1 × 105 cell/mL) and bovine serum albumin (BSA) was used as a standard by serial dilutions to establish a standard curve.13,25
Evaluation of the activity of superoxide dismutase in muscle
The collected muscle samples were thawed and individually analyzed for SOD, where 100 mg of tissue from each sample was manually macerated and homogenized with a sterile plastic pestle in 0.5 (mL) of phosphate buffer (50 mM, pH 7.8) and subsequently centrifuged at 14 000 rpm for 10 min at 4 °C.24,27 The supernatant was placed in 1.5 mL microcentrifuge tubes chilled on a bed of ice for analysis. The SOD activity was evaluated according to Suzuki28 using the xanthine/xanthine oxidase system as a constant generator of superoxide radicals, which reduce nitroblue tetrazolium to formazan, and was evaluated every 30 s for 5 min at an absorbance of 560 nm. SOD activity was defined as the amount of the enzyme required to inhibit 50 % dismutation of the superoxide radical. The results of SOD activity were expressed as U/mg of protein in the muscle.
Evaluation of the expression of immune response genes in hemocytes (RNA extraction and complementary DNA synthesis)
To determine gene expression, 500 µL of RNAlater stabilization solution (Ambion) was added to each hemocyte sample. Total RNA was obtained using TRIzol Reagent (Sigma-Aldrich®) according to the manufacturer’s instructions. RNA was quantified by spectrophotometry between 260/280 nm.8,29 The synthesis of the complementary strand (cDNA) was obtained using the Improm II protocol (Promega®), adjusting the samples to a concentration of 10 µg of RNA and storing the obtained samples (30 µL) at -20 °C.
The expression of the superoxide dismutase gene (MnSOD) was analyzed by real-time polymerase chain reaction (qPCR) using the CFX96 qPCR System (Bio-Rad), as well as the EvaGreen Sso-fast super mix reagent (Bio-Rad) and primers for specific genes.30,31 The PCR program was carried out as follows: denaturation at 95 °C for 5 min, followed by 40 cycles at 95 °C for 20 s, 60 °C for 20 s, and 72 °C for 20 s, where fluorescence was evaluated. A denaturation curve was obtained at the end of the PCR reaction to observe the specificity of the fragments and the absence of artifact formation. The primers used for gene amplification are listed in Table 1. After stability analysis, the ubiquitin and RpL8 genes were selected to normalize the data obtained using cMnSOD. The relative expression (RE) of the transcripts of the cMnSOD gene in hemocytes of L. vannamei was calculated starting from the relation between the relative quantity (QR) of each sample with the following equation, where t is the target gene (reference gene) and nf is the factor of normalization obtained from the geometric mean calculated from the QRs of the most stable reference genes (S12 and RPL8).32,33
Table 1 Primers used for the expression of the MnSOD gene by qPCR after the challenges(31, 32)
| Gen | Primer name | Sequence 5´- 3´ | Efficiency % | Fragment size (bp) |
|---|---|---|---|---|
| Ribosomal protein S12 | S12-F S12-R | GTGGAAGGAGACGTTGGTGT AGAGCCTTGACCGCTTCAT | 2.00 | 150 pb |
| Ubiquitin | UBI-F UBI-R | GGGAAGACCATCACCCTTG TCAGACAGAGTGCGACCATC | 1.98 | 146 pb |
| Ribosomal protein L8 | L8-F L8-F | GCCTAAGGTGCGTGGTGT ATTCTGCCTTGGGTCCTTCT | 2.00 | 181 pb |
| Manganese superoxide dismutase (MnSOD) | MnSOD-F MnSOD-R | ATTGGGTGAGGAACGAGGTG GGTGATGCTTTGTGTGGTGG | 2.10 | 113 pb |
Statistical analysis
The homoscedasticity and homogeneity of the data were determined using the Kolmogorov- Smirnov and Bartlett tests. A one-way analysis of variance (ANOVA) was performed using the F test to analyze the differences between the treatment and control groups. Values of P < 0.05 were considered significantly different, and Tukey’s test (HSD) was applied to identify the nature of these differences (P < 0.05). All data were evaluated using STATISTICA software (software version 2.0.).
Results
There was no significant increase in the THC content in any treatment. However, the highest average THC value was detected at 72 h post-exposure in the treatments with C. maris and laminarina compared with the control group and other treatments (Figure 1a). The laminarin treatment showed high mean THC values compared with any other treatment in almost all the samples except for 48 h post-exposure (Figure 1a). Candida maris and G. candidum showed low THC activity only at 24 h post-exposure, and their average values increased from 100 to 300 %, with twelve C. maris exhibiting these values (Figure 1a).
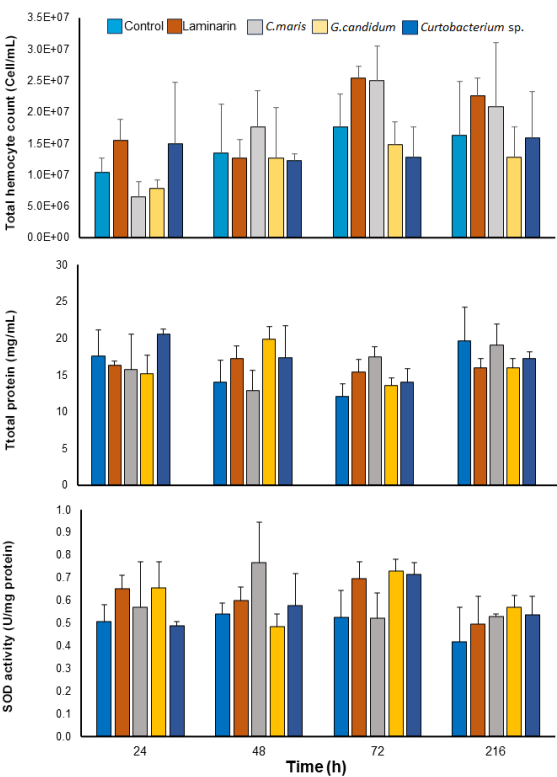
Figure 1 Hemocyte count and total protein in hemocytes, in addition to superoxide dismutase (SOD) activity in muscle in juvenile white shrimp L. vannamei exposed to the control group treatments (no treatment), laminarin (0.5 mg/mL), Candida maris, Geotrichum candidum, and Curtobacterium sp. at 1 × 106 CFU/mL each. *Significance compared with the control group at each time point.
The total protein (TP) content in the hemocytes is shown in Figure 1b. At 24 h post-exposure a significant increase (P = 0.0136) was observed in shrimp treated with Curtobacterium sp. and at 48 h post-exposure in juveniles treated with laminarin (P = 0.0418) and G. candidum (P = 0.0375) compared with the control group. At 72 h post-exposure, all treatments had higher average values of TP than the control group (Figure 1b). However, at 216 h postexposure, all treatments showed values similar to the control (untreated shrimp, Figure 1b).
Shrimp treated with laminarin showed a significant increase in the mean SOD activity of the muscle at 24 (P = 0.0435) and 72 h post-exposure (P = 0.0463) compared with the control group (Figure 1c). Candida maris generated higher average values of SOD activity at 24, 48, and 216 h post-exposure compared with the control group, showing the greatest increase at 48 h compared with the control group and other treatments. At 72 h, G. candidum and Curtobacterium sp. also registered a significant increase (P > 0.0500) in the average values of SOD activity compared to the control group (Figure 1c). At 216 h post-exposure, all treatments showed values similar to the control group (Figure 1b).
Figure 2 presents the gene expression of MnSOD in shrimp (L. vannamei) hemocytes, where a significant decrease was observed in the expression at 24 h post-exposure in all treatments compared with the control group. At 48 h post-exposure, a significant increase was observed in the gene expression of the shrimp treated with laminarin (P = 0.0273), C. maris (P = 0.0187), and Curtobacterium sp. (P = 0.0331), being at least twice the expression compared with the control group. At 72 h post-exposure, this significant increase was observed (P = 0.0357) in shrimp exposed to G. candidum and at 216 h post-exposure in shrimp exposed to C. maris (P = 0.0431). In both cases (72 and 216 h post-exposure), the gene expression of the hemocytes obtained from the shrimp exposed to these two treatments was at least twice than that of the control group. MnSOD gene expression in the hemocytes of shrimp exposed to G. candidum showed a tendency to increase up to 72 h post-exposure, decreasing subsequently.
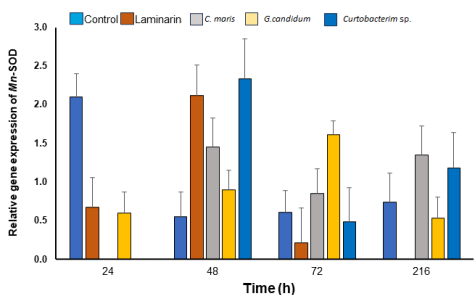
Figure 2 Relative gene expression of MnSOD in hemocytes of juvenile white shrimp L. vannamei exposed to the control group treatments (no treatment), laminarin (0.5 mg/mL), Candida maris, Geotrichum candidum, and Curtobacterium sp. at 1x106 CFU/mL each. *Significance compared with the control group at each time point (h).
Discussion
Different molecules act naturally to stimulate the defense mechanisms of organisms (immunostimulants), preventing the entry, invasion, and colonization of pathogens.8,11,34 These immunostimulant molecules come from different sources (viruses, bacteria, fungi, etc.) and are regularly supplied to cultured shrimp by different routes depending on the volume of water, density or age of cultured organisms: by direct immersion in culture water (postlarvae), by inclusion in the diet (juveniles) or by intramuscular injection (adults), favoring disease resistance against potential pathogens (without causing the microbial resistance that antibiotics generate), enhancing the survival of organisms in the face of environmental and nutritional stress.35,36
In this study, Pacific juvenile shrimp exposed by immersion at concentrations of 1 × 106 CFU/mL with microbial immunostimulants isolated from marine soil and a commercial immunostimulant (0.5 mg/mL), showed that microbial treatments increased antioxidant system and protein content in hemocytes. Ceseña et al.31 reported increased immune and antioxidant responses and resistance against experimental infections with V. parahaemolyticus in L. vannamei juveniles after exposure of shrimp to immunostimulant microorganisms included in shrimp diet and by immersion.
Adequate use of immunostimulants in terms of concentration (dose), frequency, and route of administration are relevant to obtain favorable results on the activation of the shrimp immune system. This is to avoid potential immunosuppression caused by an excessive energy loss (oxidative stress) in the organism or by null effects generated for not use an adequate specific treatment in concentration, administration method, or administration frequency of the immunostimulants. 24,36,37
The information obtained in the present study showed that the use of C. maris, G. candidum, and Curtobacterium sp. generated significant results at different periods throughout the bioassay, both in terms of hemocyte protein content and muscle SOD activity. A high level of hemocyte content was also detected at 72 and 216 h compared with the other post exposure times (24 and 48 h) used in the present study. Immunostimulants activate the immune responses of shrimp from the postlarval stage and develop the innate immunity of these crustaceans, creating a hostile environment for detected potential pathogens and promoting their timely elimination.11,34,38,39
Apines-Amar & Amar40 showed that when immunostimulants are detected by hemocytes, they initiate a chain of physiological events that prepare and strengthen the immune system in organisms against pathogens. This chain of shrimp physiological events generates binding with antibacterial peptides, phagocytosis by hemocytes, encapsulation of the detected molecule, melanization, and activation of the ProPO system.16,34,37 This produces and releases different proteins from the hemocytes to the hemolymph by exocytoses, such as prophenoloxidase, serine proteinase, peroxynectin, proteinase inhibitor, and lysozyme, in response to stimuli produced by the invasion of pathogens,41 where the destroyed materials and molecules are expelled. through the excretory system in branchial tissue, antennal gland, etc.
Hemocytes are a reliable indicator for determining and preventing diseases, as well as a sign of the physiological state of the animal. These are responsible for coagulation, release of transglutaminases, and thrombospondins, and phagocytosis and removal of foreign materials that enter the hemolymph and shrimp tissues. The detection of a foreign molecule activates these cells, stimulating the production of different extra- and intracellular products associated with the immune response and generating hemocyte proliferation and phagocytosis. This also releases proteins such as agglutinins, ProPO, antimicrobial peptides, and lysozyme that increase the total protein content in hemocytes and hemolymph.42) Some studies suggest that an increase in immunoproteins in hemocytes is induced by exposure to immunostimulants.42-44
The three experimental strains used (C. maris, G. candidum, or Curtobacterium sp.) showed immunostimulant activity in shrimp (L. vannamei), modifying the protein concentration in hemocytes at different post-challenge periods (Figure 1) and inducing significant increases in protein at 48 and 72 h postexposure compared with the control groups. Several authors13,38,45 observed similar changes and increases in the level of hemocytes and proteins when exposing L. vannamei to immunostimulants. The results show that the experimental strains used could improve the immune response and resistance to diseases of white shrimp (L. vannamei).
An important immune response of hemocytes is phagocytosis, which eliminates foreign agents; however, during this process, reactive oxygen molecules (ROS) are produced that must be neutralized.46 This neutralization is carried out by antioxidant enzymes, mainly by the SOD enzyme, which converts ROS into oxygen and hydrogen peroxide molecules. This last molecule is converted into water and oxygen by the catalase enzyme, thus neutralizing ROS and regulating cell homeostasis. The results show a significant increase in SOD when using laminarin G. candidum and Curtobacterium sp. at 72 h compared with the control group.
The SOD enzyme uses a metallic cofactor for catalysis, where manganese is one of the metals involved, thus generating MnSOD.46 García-Triana et al.47 indicated that MnSOD is an antioxidant enzyme that transforms or dismutates toxic superoxide into hydrogen peroxide and oxygen, protecting cells from the damage generated by this molecule. The MnSOD transcription level in hemocytes decreased at 24 h postexposure, with the products used, compared with the control group, followed by an increase at 48, 72, and 216 h post-exposure (Figure 2). This decrease in antioxidant expression is similar to other studies related to the use of immunostimulants, which is associated with cellular oxidative stress caused by activation of the host’s immune system at the beginning after exposure to treatments and with a posterior increase of cellular response.43,48
In addition, a decrease in shrimp immune and antioxidant response has been reported after exposure to external stressors such as pollutants, microbial infections, and hypoxia.36,48-50 Neves et al.36 reported a significant decrease in SOD enzyme activity when shrimp (Palaemonetes argentinus) was infected with Probopyrus ringueleti. Campa-Córdova et al.24 obtained a significant increase in SOD activity in L. vannamei hemocytes at 6 h post-exposure (immersion) with 𝛽-1,6 glucan (0.5 mg/mL) and a subsequent significant decrease at 72 h postexposure compared with the control group. Licona-Jain et al.35 reported increases in SOD activity in hemocytes of juvenile shrimp (L. vannamei) after the second week post-exposure to marine yeasts (1.1 % in the diet), improving shrimp resistance after exposure to a pathogenic bacterium (Vibrio parahaemolyticus).
Conclusions
Based on the results observed for the three microorganisms obtained from the marine environment, these showed that juvenile Pacific shrimp (L. vannamei) exposed to a concentration of 1 × 106 CFU/mL for 48 h present an increase in the protein content in circulating hemocytes, in addition to an increase in the enzymatic activity and gene expression of superoxide dismutase. This study shows the importance of increasing studies to clarify the issue.











 nueva página del texto (beta)
nueva página del texto (beta)



