Introduction
The prolapse of the mitral valve (Barlow syndrome) is defined as the displacement of at least 2 mm of the mitral valve during systole secondary to myxomatous degeneration of the valve, resulting in a thickening of 5 mm during diastole1, it is a common cardiac pathology whose frequency is estimated between 1% and 3% of the general population, predominantly in women2-5. Its high lethality is related to the presence of severe mitral insufficiency with a reduced left ventricular ejection fraction (LVEF) (VEF < 60%) in whom surgical treatment is preferred, with valve repair being the one of choice6, it is also associated with ventricular arrhythmias and sudden cardiac death (SCD)7. In 1963, John Brereton Barlow described it as the presence of a mesosystolic click accompanied by a systolic murmur, associated with the ripple and regurgitation of the valves8, in which specific clinical manifestations are palpitations, atypical thoracic pain, dyspnea, headache, amaurosis (sudden blindness), anxiety disorders, and electrocardiographic abnormalities9. Next, to expand knowledge about this pathology, a clinical case is exposed whose clinical manifestations make it difficult for health professionals to diagnose.
Case report
History and current disease
Maternal and paternal grandparents with systemic arterial hypertension, maternal grandfather ended up by a cerebrovascular disease, and maternal grandmother ended up due to complications of type 2 diabetes mellitus.
A 16-year-old female patient who enters the emergency room in 2013 presenting blood pressure (BP) 170/100 mmHg, with palpitations that limit her physical activity accompanied by dyspnea, vertigo, blurred vision, imminent death sensation, tinnitus, anxiety, and tremor, so she was treated with antihypertensives (Captopril and Losartan), she is later discharged. The following month after multiple hypertensive crises and exacerbation of symptoms, she is admitted to the internal medicine service where metabolic and renal causes are ruled out with laboratory studies, abdominal ultrasound, and renal and pelvic Doppler, abdominal-pelvic axial computed tomography (CT) was performed, ruling out probable pheochromocytoma, as well as disorders of affection, anxiety, and psychotic episodes by psychiatry service, so she is referred to the cardiology service, where the study protocol begins.
Vital signs
BP: 170/100 mmHg, heart rate (HR): 200 lpm, respiratory rate (RR): 17 rpm, T°: 36.5°C, size: 1.66 m, weight: 85 kg, and body mass index: 30.8 kg/m2.
Physical examination
Female of apparent to chronological age, oriented in person, time and space, cooperative, body symmetrical, endomorph, anxious facies, precordial area: There is the presence of a reinforced noise and a systolic click separated from the first noise, followed by a mesosystolic murmur, examination of the head, neck, abdomen, superior and inferior limbs, and genitals without significant abnormalities.
Complementary tests
The echocardiogram reports LVEF of 79% and mild prolapse of the anterior valve of the mitral valve. Two-dimensional, M-mode echocardiogram and pulsed wave Doppler, continuous wave and color-coded where discreetly thickened mitral valve is evident, elongated with mild septal valve prolapse and minimal heart failure, as shown in figures 1 and 2. Simple and contrast abdominal CT reports no evidence of abnormalities in the adrenal glands. Simple and contrasted aorta CT from supra-aortic trunks to iliac bifurcation with axial and sagittal cuts, projections of maximum intensity, as well as in 3D reconstruction reports no morphologic abnormalities. Electrocardiogram showed sinus tachycardia without alterations. Holter monitoring: sinus rhythm with the presence of two supraventricular extrasystoles during the study isolated and infrequent with a maximum R-R of 1.5 s.

Figure 1 Two-dimensional echocardiogram. When analyzing real-time mobility on the parasternal axis, prolapse of the anterior valve of the mitral valve is observed.

Figure 2 The transmitral flow is seen, which is constant and has a normal I/A ratio, indicating that the diastolic function is normal.
Adrenal gammagram MIBG 131 (iodo-131-metaiodobenzylguanidine) without alterations. Fractionated metanephrines urine of 24 h total: 62.10 pg/24 h, normetanephrine: 34.5 pg/24 h, metanephrine 27.6 pg/24 h, triiodothyronine (T3) 120 ng/dL, tetraiodothyronine (T4L) 0.9 ng/dL, thyroid-stimulating hormone 1.60 mIU/mL, cortisol 25.2 mg/dL, insulinotropic growth factor 1: 511 ng/mL, growth hormone 2.5 ng/mL, insulin 19.7 mIU/mL, electrolytes, and arterial gases without abnormalities.
Evolution
It begins with a study protocol to rule out causes of secondary hypertension, so antihypertensive treatment is suspended to assess the behavior of BP figures, with subsequent development of hypertensive crisis accompanied by sinus tachycardia and syncope.
According to the previous results, secondary causes of arterial hypertension are ruled out since there is no evidence to lead to another pathology, so it is concluded that she suffers from primary hypertension. Subsequently, she was referred to the endocrinology service where, together with the cardiology service, they diagnosed Barlow syndrome and primary arterial hypertension, requiring surveillance by the second level of care, with treatment based on propranolol, prazosin, and propafenone, being discharged at the third level of care.
At present, the patient is hemodynamically stable, in an outpatient cardiology consultation, follow-up is carried out with laboratory studies, electrocardiogram, M-mode, two-dimensional and Doppler echocardiography, 24-h Holter study, and BP auto-monitoring (BPAM). The results of the M mode, two-dimensional, and Doppler mode echocardiogram in the mitral valves demonstrated an E-F slope of 163 mm/s, D-E distance of 19 mm, E-septum distance of 5 mm, and thickness of the anterior valve of 4 mm, in the Doppler mode a slight insufficiency is observed, with thickened, elongated, and redundant valves that condition a slight prolapse of the anterior valve, diastolic thickness of the posterior wall of 7 mm, and ejection fraction were calculated by Teicholz method with 78% of ejection fraction. Circumferential shortening fraction of 42%, tri cuspid ring velocity of 14 cm/sec, basal of 25 mm, medium segment of 24 mm, and length of 57 mm. Normal global and segmental mobility at rest was found. The systolic pressure of the pulmonary artery was 28 mmHg. There were no atrial dilation, or evidence of masses, thrombi or shunt.
According to the results of the 24-h Holter study, sinus rhythm is observed with maximum PR 156 ms to 51 bpm, minimum 172 ms to 154 bpm, which is conducted with narrow QRS of 84 ms throughout the study, minimum QT 383 ms to 154 ms, maximum of 444 ms to 51 bpm, and QT corrected by Bazett 418 and 510 ms, respectively. Eight supraventricular ectopic beats were documented, organized in isolation, with no episodes of supraventricular tachycardia. The longest RR interval was 1.28 s at 23:55:53 h, during physiological sleep, corresponding to sinus bradycardia, with the conclusion of normal HR.
Regarding the 24-h BPAM, the average vigil state BP was 124/77 mmHg, during sleep, it was 107/55 mmHg, and the average was 118/70 mmHg in 24 h, observing BP figures within normal parameters according to the ESH/ESC 2018 Clinical Practice Guidelines.
Electrocardiogram at a speed of 25 mm/sec demonstrates sinus rhythm, without the presence of arrhythmias.
Laboratory studies without abnormalities
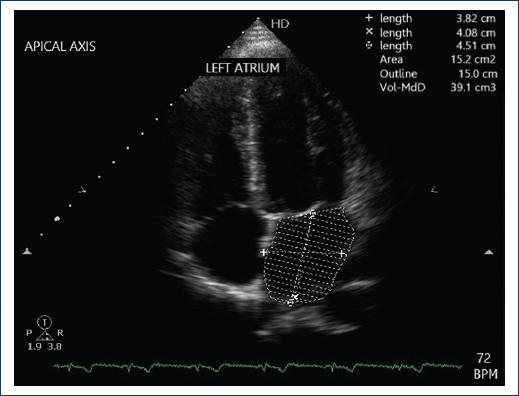
As can be seen in the patient’s follow-up, she has not shown morphological and electrocardiographic changes despite a time of approximately 10 years from the onset of the underlying pathology, as shown in figure 3.
Discussion
Mitral valve prolapse (MVP) is one of the most common valve abnormalities, where 11.7% suffer from SCD (MCS)8 because the movement of the valve apparatus and their respective papillary muscles favors the generation of early ventricular complexes and polymorphic ventricular tachycardia, with the subsequent generation of ventricular fibrillation10. The clinical finding of a systolic click accompanied by a mesosystolic murmur is usually sufficient for the diagnostic suspicion of PVM; however, this diagnosis is complicated among health professionals, whether cardiologists or not. Most people with MVP do not know their condition, because they remain asymptomatic throughout their lives, and a small proportion have non-specific symptoms, difficult to correlate with Barlow syndrome.
Echocardiographic criteria classify prolapse into two types, the primary because it is a degenerative and hereditary disease (the most recent genetic cause of PVM was found in the DZIP1 gene associated with ciliogenesis; in addition, pathological variants have been demonstrated in six different genes (HCN4, DSP, TTN, MYH6, TRPS1, and TMEM in about 11% of the patients) and are associated with 18, 13, and 15 trisomy, Marfan syndrome, hereditary colorectal polyposis, Ehlers-Danlos syndrome, Williams-Beuren syndrome, and elastic pseudoxanthoma)4,11 and with ischemic cardiopathy with the consequence of papillary muscle dysfunction and dilatation of the mitral valve. The importance of performing physical examination and 2D12 transesophageal echocardiography, considered gold standards for this pathology, is highlighted. In these studies, the presence of regurgitation, elongated, and thickened valves can be observed, which allow the early diagnosis of Barlow syndrome to avoid complications such as SCD13.
In addition, it is necessary to perform complementary studies such as electrocardiogram, Holter, stress test, genetic studies, cardiac CT, and cardiac magnetic resonance (CMR)14 since they have a high sensitivity and specificity, so they allow an adequate follow-up and determine a long-term prognosis in the quality of life of our patients.
In this clinical case, the patient was for 9 years in treatment with propranolol, prazosin, and propafenone, without morphological modifications from the diagnosis of the disease in 2013 until today; however, the patient reports having isolated periods of palpitations and a sensation of extrasystoles, according to the 2020 ACC/AHA Guide for the Management of Patients with Heart Valve Disease, should be treated with an antiarrhythmic and an aldosterone antagonist, like spironolactone, preferring this, due to its synergistic effect with the inhibition of myocardial fibrosis6, based on the current guidelines and the aforementioned studies, it is decided to change treatment based on spironolactone and propafenone to reduce the complications associated with this pathology such as hypertrophy and fibrosis related to cardiac remodeling, severe mitral insufficiency15, and life-threatening arrhythmias such as ventricular fibrillation and SCD10. The iet of mitral regurgitation will generate frequent atrial arrhythmias. Arrhythmia generates dilation and dilation generates arrhythmia, so cutting this vicious cycle with an antiarrhythmic may reduce the possibility of generating arrhythmia and dilation of the cavities in the long term.
Conclusions
Barlow syndrome is a pathology that, despite being common, has low diagnostic accuracy among non-cardiologist health professionals, so the importance of carrying out complete physical examination and complementary studies facilitating its early detection and avoiding complications such as malignant ventricular arrhythmias that can lead to SCD is highlighted. Although the two-dimensional transesophageal echocar- diogram is considered the gold standard for the diagnsosis of MVP, the cardiac magnetic resonance (CMR) has a sensitivity and specificity of 100%16, so this can be considered the most effective study for the diagnosis of MVP for any physician even if is not cardiologist.
Regarding the diagnostic difficulty of the clinical case presented, it is very important to corroborate the information. The main cause of the error in the diagnosis is poor knowledge of the pathology.
This article was made for the purpose of early detecting patients who have a symptomatology compatible with Barlow syndrome to prevent progression to major complications that are mostly lethal.











 nueva página del texto (beta)
nueva página del texto (beta)