Introduction
Coffee residues, including spent coffee grounds (SCG), represent a high volume agro-industrial waste worldwide, due to the great demand of the beverage consumption. This waste is generated by coffee consumers after the beverage preparation (instant coffee) and has been associated with toxic and environmental concerns due to their high organic matter content and certain chemical components (Mussatto et al. 2011). In contrast, it has been reported that the aforementioned coffee residue is an important source of soluble carbohydrates like monosaccharides, oligosaccharides, and polysaccharides, as well as insoluble polysaccharides including hemicelluloses and cellulose (ß(1-4)mannan). Also, lipids (mainly palmitic-16:0 and linoleic acid-18:2 fatty acids), minerals (potassium, magnesium, sodium, iron, zinc, copper, among others), nitrogen compounds (free amino acids, proteins, caffeine, and trigonelline), organic acids (citric, malic, and quinic), and some phenolic compounds have been reported (Campos-Vega et al. 2015, Mussatto et al. 2011).
Phenolic compounds present in plant residues, like phenolic acids and flavonoids, have been considered bioactive compounds with multiple biological properties such as antimicrobial, anti-inflammatory, cardioprotective, antioxidant, among others. Thus, in recent years many bioactive compounds have been recovered through several conventional extraction methods (Gomes-Araújo et al. 2021). However, recently the production or extraction of phenolic compounds using fungal fermentation process from agro-industrial residues has received great attention due to the potential uses in pharmaceutical and food industries. For example, a previous study evaluated the potential effect of solid-state fermentation using Aspergillus oryzae and Rhizopus oligosporus as fungal strains, and wheat, brown rice, maize, and oat as substrates. This study demonstrate that fungal fermentation increases the antirradical DPPH• and ABTS•+ potential of the cereals (Bhanja Dey and Kuhad 2014). Also, the effect of solid-state fermentation using A. oryzae over the release of phenolic compounds from brewer´s spent grain and demonstrated an increase in the extraction of total phenolic compounds like phenolic acids and flavonoids, as well as antiradical activity after three days of fermentation (da Costa Maia et al. 2020).
In the same way, it has been reported an increase of total phenolic and flavonoids contents from orange peel waste after 54 h, during submerged-state fermentation using Aspergillus fumigatus (Sepúlveda et al. 2020). Also, it has been reported an increase of antioxidant activity and antibacterial properties against foodborne pathogens (Staphylococcus aureus, Escherichia coli O157:H7, among others) of extracts obtained from fermented residues (pine apple peel, mango peel, plantain peel, walnut husk wastes, groundnut shell, and coconut husk), using Pleurotus pulmonarius in submerged culture (Ogidi et al. 2020). It has been demonstrated an increase of phenolic components release and antioxidant activity of rice bran, a byproduct of the rice industry milling, fermented with A. oryzae under solid-state fermentation (Punia et al. 2020). In another study, it was compared the bioactive compounds extraction from grape pomace by solid-state and submerged fermentation, using A. niger as fungal strain, and the results revealed that both fermentation systems increase bioactive compounds release (Amaya-Chantaca et al. 2022).
Nevertheless, the use of fungal fermentation as potential alternative to extract bioactive compounds from coffee residues, to be used as possible antioxidant additives are not well studied.
Therefore, the aim of this study was to evaluate the antioxidant effect of the aqueous extract obtained from SCG, under submerged-state fermentation using Pleurotus ostreatus.
Material and methods
Raw material
The coffee residue (SCG) was collected from a local commercial supplier (CAFFENIO®, Hermosillo, Mexico), dried at 60 °C (drying oven Yamato DX402, Tokyo, Japan) until 10 % of moisture content was reach, and sterilized at 121 °C for 20 min (high-pressure steam sterilizer Yamato SM300, Tokyo, Japan). Furthermore, filamentous Pleurotus ostreatus strain, belonging to the fungal collection of the Plant-based Food Technology Department from the Food Research and Development Center (Hermosillo, Mexico), were grown on petri dishes containing PDA medium at 25 °C for 5 days (incubator Yamato IC602, Tokyo, Japan), until mycelia fully covered the surface, and stored at 4 °C.
Culture medium and fermentation conditions
The fermentation medium used for substrate moistening was sterilized at 121 °C for 20 min and composed as follows: glucose (20 g/L), yeast extract (5 g/L), KH2PO4 (1 g/L), MgSO4 7H2O (0.5 g/L), and ascorbic acid (0.05 g/L). The pH was adjusted to 5.4 by addition of either HCl (0.1 N) or NaOH (2.5 M). Shake flask culture was carried out in 250 mL Erlenmeyer flasks containing 100 mL of the medium and SCG (0, 5, and 10 %), without or with P. ostreatus mycelium. The flasks were incubated at 150 rpm at 32 °C (rotary incubator shaker MaxQTM 5000, Fisher Scientific, Nepean, Canada) for 10 d, in the dark.
Extract preparation
The culture media (aqueous solution) was homogenized at 10,000 rpm for 30 s (Ultraturrax T25, IKA®, Staufen, Germany), filtered (Whatman 1 filter paper) under vacuum (vacuum pump MVP 6, Soosung Vacuum Co., Ldt., Jeju, Korea), and dried (freeze dryer Yamato DC401, Tokyo, Japan). The obtained aqueous extract was stored at -20 °C in the dark, until analysis (Liu et al. 2018).
Phytochemical screening
The qualitative phytochemical analysis of the aqueous extract was carried out according to standard methods reported previously (Nguyen et al. 2022, Vargas-Sánchez et al. 2023). A total of 0.5 g of the aqueous extract was homogenized with 10 mL of distilled water at 10,000 rpm for 1 min (vortex mixer, Fisher ScientificTM, CA, USA) and filtered (Whatman 1 filter paper), in order to extract phytochemicals (stock solution).
For carbohydrates analysis (Phenol-sulfuric acid test) the stock solution (2 mL) was mixed with 1 mL of aqueous phenol (1 %, v/v) and 1 mL of H2SO4 concentrated, and incubated (100 °C for 5 min, in the dark). Reddish-brown precipitate indicates a positive result. For phenols analysis (Ferric chloride test) the stock solution (2 mL), previously incubated at 100 °C for 10 min and filtered, was mixed with 2 mL of FeCl3 solution (0.1 %, w/v). Blue-black precipitate indicated a positive result.
For flavonoids analysis (Shinoda test) few pieces of magnesium ribbon and 0.1 mL of HCl concentrated were carefully added to 1 mL of the stock solution. Red color formation indicates a positive result. For chlorogenic acid analysis (Sodium nitrite test) the stock solution (1 mL) was mixed with 1 mL of urea (0.17 M), 1 mL of glacial acetic acid (0.1 M), and 2.5 mL of distilled water. After, 2.5 mL of NaNO2 (0.14 M) and 2.5 mL NaOH were added (0.5 M). Red color formation indicates a positive result.
Phytochemical content
The total carbohydrate content was determined by the phenol-sulfuric acid procedure (Albalasmeh et al. 2013). An aliquot of the extract (50 µL, at 5 mg/mL) was added to 96-well clear microplate and mixed with 25 µL of aqueous phenol solution (5 % v/v) and 125 µL of concentrated H2SO4. The resultant mixture was incubated at room temperature (25 °C) for 10 min, in the dark. The absorbance was measured at 490 nm in a spectrophotometer (Multiskan FC UV-Vis, Thermo Scientific, Vantaa, Filand). Total carbohydrate content values were calculated from a standard curve of glucose (62.5 to 1,000 µg/mL; y = 0.6533x; r2 =0.9990), and results were expressed as mg of glucose equivalents per g of dried extract (mg GE/g).
The total phenolic content was determined by the Folin-Ciocalteu’s method, reported previously (Bibi et al. 2022). An aliquot of the extract (20 µL, at 5 mg/mL) was homogenized with 160 µL of distilled water, 60 µL of Na2CO3 (7 %, w/v), and 40 µL of Folin-Ciocalteu’s reagent (2 M). The resultant mixture was incubated at 25 °C for 1 h, in the dark. The absorbance was measured at 750 nm. Total phenolic content values were calculated from a standard curve of gallic acid (62.5 to 1,000 µg/mL; y = 0.4933x; r2 =0.9996), and results were expressed as mg of gallic acid equivalents per g of dried extract (mg GAE/g).
The total flavonoids content was determined by the NaNO2-Al(NO3)3-NaOH procedure reported previously (Bibi et al. 2022). An aliquot of the extract (500 µL, at 5 mg/mL) was mixed with 1 mL of NaNO2 (5 %, w/v), 1 mL of AlCl3 (10 %, w/v), and 10 mL of NaOH (1 M). The resultant mixture was adjusted to 25 mL with ethanol (70 %), and incubated at 25 °C for 15 min (water bath Yamato BM510, Tokyo, Japan), in the dark. The absorbance was measured at 510 nm. Total flavonoids content values were calculated from a standard curve of quercetin (62.5 to 1,000 µg/mL; y = 0.0299x; r2 =0.9995), and results were expressed as mg of quercetin equivalent per g (mg QE/g).
The caffeoylquinic acid content was determined as previously described (Nguyen et al. 2022). An aliquot of the extract (100 µL, at 5 mg/mL) was homogenized with 200 µL of urea (0.17 M), 200 µL of glacial acetic acid (0.1 M), and 500 µL of distilled water. The resultant mixture was mixed with 500 µL of NaNO2 (0.14 M) and 500 µL of NaOH (0.5 M) and centrifuged at 2,250 g at 4 °C for 10 min, in the dark (Sorvall ST18R, Thermo Fisher Scientific, Waltham, USA). The absorbance was measured at 510 nm. Total caffeoylquinic acid content values were calculated from a standard curve of chlorogenic acid (62.5 to 1,000 µg/mL; y = 0.1201x; r2 =0.9990), and results were expressed as mg of caffeoylquinic acid equivalents per g (mg CQA/g).
Antioxidant activity
The free-radical scavenging activity (FRSA) was determined according to the DPPH• method, reported previously (Bouhlal et al. 2020). An aliquot of extract (100 µL, at 100 µg/mL) was homogenized with 100 µL of DPPH• solution (300 µmol), and incubated at 25 °C for 30 min, in the dark. The absorbance was measured at 520 nm, and results were expressed as inhibition percentage and calculated as follow:
The radical-cation scavenging activity (RCSA) was determined according to the ABTS•+ method, reported previously (Bouhlal et al. 2020). An aliquot of extract (20 µL, at 100 µg/mL) was homogenized with 980 µL of ABTS•+ solution (absorbance 0.8 nm, in ethanol), and incubated at 25 °C for 30 min, in the dark. The absorbance was measured at 730 nm, and results were expressed as inhibition percentage and calculated as follow:
The reducing power ability (RPA) was determined by the Ferricyanide/Prussian blue method (Berker et al. 2010). An aliquot of extract (100 µL, at 5 mg/mL) was homogenized with 300 µL of phosphate buffer (0.2 M, pH 6.6) and 300 µL of potassium ferricyanide (1 %, w/v). The resultant solution was incubated at 50 °C for 20 min in a water bath, under dark. After cooling at 25 °C for 10 min, the mixture was homogenized with 300 µL of trichloroacetic acid (10 %, w/v) and centrifuged (4,200 g at 4 °C for 10 min). Then, 100 µL of the supernatant were mixed with 100 µL of distilled water and 250 µL of ferric chloride (0.1 %, w/v). The absorbance was measured at 700 nm, and results were expressed as absorbance increase at the same wavelength.
Ferric-reducing antioxidant power (FRAP) was determined (Berker et al. 2010). An aliquot of extract (20 µL, at 5 mg/mL) was homogenized with 150 µL of FRAP solution [10:1:1, 300 mM buffer sodium acetate in glacial acetic acid at pH 3.6 and 10 mM 4,4,6-tripyridyl-S-triazine (TPZ) in 40 nM HCl and 20 mM FeCl3]. The reaction mixture was incubated at 25 °C for 8 min, in the dark. The absorbance was measured at 595 nm, and results were expressed as mg of Fe2+ equivalents per g (mg Fe2+/g). Lipid oxidation (LOX) was determined by the thiobarbituric acid reactive substances (TBARS) method previously reported (Kim et al. 2016), with slight modifications. Meat homogenates were obtained homogenizing (4,500 rpm at 4 °C for 1 min) pork meat with distilled water (1:10, w/v) and the respective antioxidants at 500 ppm. The resultant solution was incubated for 60 min at 65 °C in a water bath. Then, meat homogenates (0.5 mL) were mixed with 1 mL of TCA solution (10 %, w/v). After, 1 mL of the resultant filtered solution (Whatman 1 filter paper) was homogenized with 1 mL of TBA solution (0.02 M) and placed in a water bath (97 °C for 20 min), and subsequently cooled. The absorbance was measured at 531 nm, and results expressed as mg of malondialdehyde per kg of meat (mg MDA/kg).
Statistical analysis
Mean ± standard deviation values were used as descriptive statistics. All data were obtained from three independent experimental trials (with three replications). Data of phytochemical composition and antioxidant activity were submitted to one-way analysis of variance (ANOVA) with the fixed effect of treatment. While data of meat extract measurement were subjected to a twoway factorial ANOVA, which treatment and storage time were considered the main effects in the model. A Tukey-Kramer multiple comparison test was performed for meat separation (p < 0.05). Furthermore, a Pearson´s correlation analysis and a principal component analysis were performed to determine the relationships among the analyzed variables (SPSS, version 21).
Results
Chemical composition
Table 1 reports the preliminary phytochemical profile of the aqueous extracts obtained from SCG after fungal fermentation process. The results showed that phytochemicals like carbohydrates, polyphenols, and flavonoids were highly present in the in the aqueous extract obtained from SCG fermented with P. ostreatus, at the highest substrate concentration 10 % (SCG 10 % + PO). While caffeoylquinic acid was highly present in SCG 5 % + PO and SCG 10 % + PO. In addition, the aqueous extract obtained from SCG 0 % + PO, as well as the aqueous extracts from fermented samples without P. ostreatus, showed slight presence of these compounds. Furthermore, polyphenols, flavonoids, and caffeoylquinic acid were not detected in the control sample (SCG 0 %).
Table 1 Effect of liquid culture fermentation of SCG by P. ostreatus on the phytochemical profile of their aqueous extract
| Extracts | Carbohydrates | Polyphenols | Flavonoids | Caffeoylquinic acid |
| SCG 0 % | + | + | + | + |
| SCG 5 % | + | + | + | + |
| SCG 10 % | + | + | + | + |
| SCG 0 % + PO | + | + | + | + |
| SCG 5 % + PO | ++ | ++ | ++ | +++ |
| SCG 10 % + PO | +++ | +++ | +++ | +++ |
SCG, spent coffee grounds. PO, P. ostreatus. (-), absent. (+), slightly present. (++), moderately present. (+++), highly present.
Table 2 reports the phytochemical content of the aqueous extracts obtained from SCG after fungal fermentation process. The results showed that the aqueous extract obtained from SCG fermented with P. ostreatus, at the highest substrate concentration (SCG 10 % + PO), showed the highest (p < 0.05) carbohydrates, polyphenols, flavonoids, and caffeoylquinic acid content in comparison to fermented samples without P. ostreatus.
Table 2 Effect of liquid culture fermentation of SCG by P. ostreatus on the phytochemical content of their aqueous extract
| Extracts | Carbohydrates (mg GE/g) | Polyphenols (mg GAE/g) | Flavonoids (mg QE/g) | Caffeoylquinic acid (mg CQA/g) |
| SCG 0 % | 21.88 ± 0.78a | 1.46 ± 0.25a | 0.76 ± 0.05a | 2.01 ± 0.50a |
| SCG 5 % | 23.85 ± 0.30b | 8.68 ± 1.09b | 4.07 ± 0.77b | 56.52 ± 2.30c |
| SCG 10 % | 23.84 ± 0.31b | 9.62 ± 0.82b | 3.75 ± 0.92b | 92.93 ± 2.19d |
| SCG 0 % + PO | 28.62 ± 1.73c | 8.07 ± 0.35b | 4.59 ± 0.22b | 31.88 ± 1.79b |
| SCG 5 % + PO | 87.98 ± 1.96d | 19.23 ± 2.41c | 9.99 ± 1.30c | 226.36 ± 1.55e |
| SCG 10 % + PO | 101.46 ± 0.75e | 24.75 ± 2.01d | 12.52 ± 0.32d | 329.98 ± 4.72f |
Values are expressed as mean ± standard deviation. SCG, spent coffee grounds. PO, P. ostreatus. Means with different superscripts (a-f) among samples indicate significant differences with the Tukey test (p < 0.05).
Antiradical and reducing power properties
Table 3 reports the antioxidant properties of the aqueous extracts obtained from SCG after fungal fermentation process. The results indicate that the highest (p < 0.05) antiradical activity (FRSA and RCSA) was showed in the aqueous extract obtained from SCG fermented with P. ostreatus, at the highest substrate concentration (SCG 10 % + PO). Regard reducing power properties, the results indicate that aqueous extracts obtained from SCG fermented with P. ostreatus showed higher (p < 0.05) RPA and FRAP values than fermented samples without P. ostreatus, in concentration dependence.
Table 3 Effect of liquid culture fermentation of SCG by P. ostreatus on the antioxidant activity of their aqueous extract
| Extracts | FRSA (% Inhibition) | RCSA (% Inhibition) | RPA (abs) | FRAP (mg Fe2+/g) |
| SCG 0 % | 27.78 ± 0.59a | 52.94 ± 1.23a | 0.42 ± 0.01a | 3.25 ± 0.04a |
| SCG 5 % | 56.93 ± 0.61d | 85.23 ± 0.43c | 0.53 ± 0.01b | 9.72 ± 0.04c |
| SCG 10 % | 63.96 ± 0.32e | 85.55 ± 0.98c | 0.55 ± 0.02b | 10.95 ± 0.10d |
| SCG 0 % + PO | 31.47 ± 0.95b | 78.98 ± 1.27b | 1.28 ± 0.02c | 4.01 ± 0.17b |
| SCG 5 % + PO | 43.22 ± 1.83c | 85.32 ± 0.50c | 1.30 ± 0.03c | 16.64 ± 0.71e |
| SCG 10 % + PO | 71.59 ± 0.58f | 88.89 ± 0.35d | 1.57 ± 0.01d | 28.50 ± 0.95f |
Values are expressed as mean ± standard deviation. SCG, spent coffee grounds. PO, P. ostreatus. FRSA, free-radical scavenging activity (DPPH• method). RCSA, radical-cation scavenging activity (ABTS•+ method). RPA, reducing power ability. FRAP, ferric-reducing antioxidant power. Means with different superscripts (a-f) among samples indicate significant differences with the Tukey test (p < 0.05).
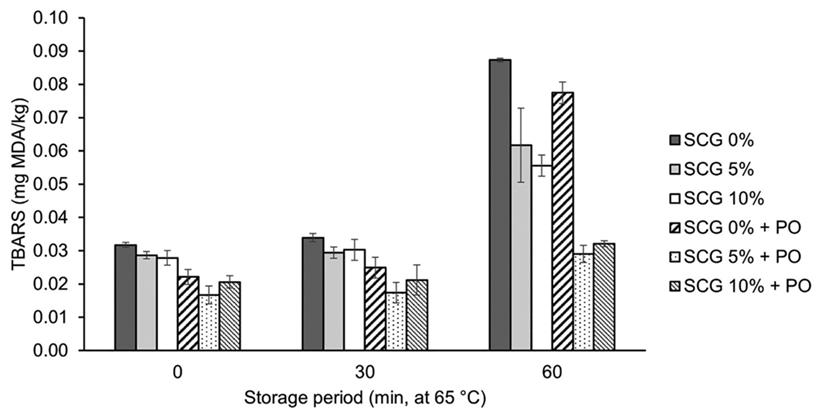
Lipid oxidation inhibition
Figure 1 reports the effect of the inclusion of the aqueous extracts obtained from SCG after fungal fermentation process and thermal storage time on lipid oxidation of pork meat homogenates. The results indicate that lipid oxidation was significantly (p < 0.05) affected by treatment x thermal storage period. At the beginning of storage, lipid oxidation of meat homogenates was significantly (p < 0.05) reduced by the aqueous extracts obtained by fungal fermentation. Although lipid oxidation values increased (p < 0.05) during the whole period for all treatments, at the end of storage, meat homogenates treated with the aqueous extracts obtained from SCG and fermented with P. ostreatus (SCG 5 % + PO = SCG 10 % + PO) showed the lowest (p < 0.05) lipid oxidation values when compared with another treatments.
Pearson’s correlation analysis
Table 4 reports the association between treatments and analyzed variables. The results of Pearson’s correlation analysis indicate that FRAP values showed a high positive correlation with total carbohydrates, polyphenols, flavonoids and caffeoylquinic acid content (0.893, 0.951, 0.921 and 0.977, respectively). While, RPA values showed a high positive correlation with total carbohydrates, polyphenols and flavonoids content (0.820, 0.813 and 0.867, respectively). In contrast, FRSA and RCSA values showed a slight relationship with the metabolites content (<0.75). In addition, TBARS values showed a negative correlation with the metabolites content.
Table 4 Correlation between metabolites and antioxidant activity of the treatments
| Carb. | Polyph. | Flav. | CQAs | FRSA | RCSA | RPA | FRAP | TBARS | |
| Carb. | 1.000 | 0.944 | 0.962 | 0.961 | 0.405 | 0.471 | 0.820 | 0.893 | -0.394 |
| Polyph. | 1.000 | 0.992 | 0.982 | 0.632 | 0.724 | 0.813 | 0.951 | -0.647 | |
| Flav. | 1.000 | 0.968 | 0.539 | 0.678 | 0.867 | 0.921 | -0.615 | ||
| CQAs | 1.000 | 0.638 | 0.612 | 0.751 | 0.977 | -0.508 | |||
| FRSA | 1.000 | 0.743 | 0.184 | 0.763 | -0.607 | ||||
| RCSA | 1.000 | 0.509 | 0.635 | -0.980 | |||||
| RPA | 1.000 | 0.654 | -0.535 | ||||||
| FRAP | 1.000 | -0.507 | |||||||
| TBARS | 1.000 |
Carb., carbohydrates. Polyph., polyphenols. Flav., flavonoids. CQAs, caffeoylquinic acid. FRSA, free radical scavenging activity. RCSA, radical cation scavenging activity. RPA, reducing power ability. FRAP, ferric-reducing antioxidant power. TBARS, thiobarbituric acid reactive substances.
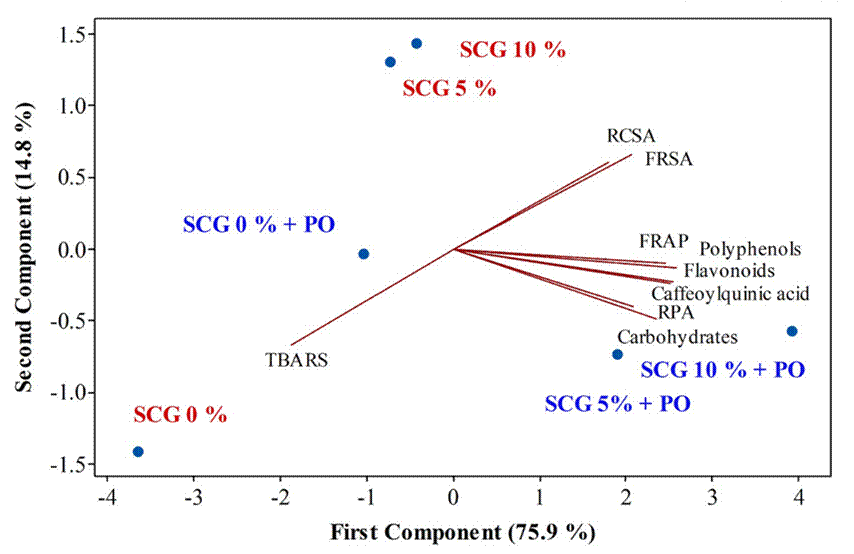
Principal component analysis
Figure 2 reports the principal component analysis carried out in order to evaluate the differences between treatments and analyzed variables. The first and second component showed a variance of 75.9 % and 14.8 %, respectively; thus, an accumulative of 90.7 % of the total variation was explained by the two components. In addition, the results showed a separation of analyzed treatment and antioxidant activity (p < 0.05), for example, SCG 5 % + PO and SCG + PO treatments showed highest relationship with total carbohydrates, polyphenols, flavonoids, and caffeoylquinic acid, as well as FRAP and RPA values.
Discussion
Chemical composition
Phytochemicals from the Greek (phyton = plant), including alkaloids, glycosides, terpenes, terpenoids, saponins, steroids, phenolics, tannins, flavonoids, among others, are chemical compounds presents in plants. These compounds play an important role in plant growth supporting against competitors, pollution, UV-light, diseases and another stress plant factors. In addition, they are associated with human health benefits and with some biological properties of interest for the food industry. However, the bioactivity of natural extracts depends on the constituent’s type after separate the phytochemicals from the plant material using extraction techniques (Shaikh and Patil, 2020). This composition can be affected by pre-extraction factors like type of plant material and processing conditions (drying, milling, among others), as well as extraction related factors including solvent type, solvent-solid ratio, time, and the employed method. Regard extraction methods, decoction, hot continuous (Soxhlet), infusion, maceration, and percolation, are commonly employed as conventional extraction methods, while, microwave-assisted, ultrasonic-assisted, subcritical water, supercritical fluid, and membrane technology are considered as unconventional extraction methods. In addition, enzymatic and fermentation extraction process have been used as biotechnology extraction methods (Shaikh and Patil 2020, Gomes-Araújo et al. 2021).
In this context, a preliminary phytochemical screening showed the presence of carbohydrates, phenols, and flavonoids in the ethanol extract obtained from prepared coffee bean powder (Canephora robusta) using Soxhlet extraction method (Jankuti et al. 2020). In another study, phenolic components were also reported in aqueous and ethanol extracts from SCG, using percolation extraction and a solvent-solid ratio of 1:10 (Kim et al. 2016). While, phenolic and caffeoylquinic acid were identified in an aqueous extract from SCG, using percolation extraction and a solvent-solid ratio of 1:2 approximately (Belviso et al. 2014). Also, phenolic compounds were reported in SCG extracts (C. arabica) using hexane, water, and ethanol as solvent extraction (solvent-solid ratio of 1:7 approx.), and Soxhlet, ultrasound, and accelerated solvent as extraction methods (Mitraka et al. 2021).
Regard biotechnological extraction methods, Machado et al. (2012) demonstrated that phenolic compounds can be extracted from SCG by solid-state fermentation using different fungal strain, including Aspergillus sp., Mucor sp., and Penicillium sp. In another investigation, da Costa Maia et al. (2020) reported a phenolic compounds extraction from SCG by solid-state fermentation using Aspergillus oryzae; while trans-ferulic acid, p-coumaric acid, ferulic acid, protocatechuic acid, genticic acid 3,7-dimethylquercetin, phlorizine, and naringenin 7-O-glucoside were the identified phenolic compounds. In another study, Han et al. (2021) demonstrated a release of phenolic compounds from SCG by submerged-state fermentation using Cordyceps sinensis. Although there is limited information on the use of submerged-culture fermentation using P. ostreatus as potential alternative to extract bioactive compounds from coffee residues, a previous investigation was performed to extract bioactive components from SCG by submerged fermentation using P. ostreatus (Carrasco-Cabrera et al. 2019). In this context, the findings obtained from our study highlight that the aqueous extracts from SCG fermented with P. ostreatus is a promising source of bioactive compounds.
Antiradical and reducing power properties
Moreover, it has been demonstrated that phytochemicals are associated with the antioxidant activity of extracts obtained from coffee residues (Mussatto et al. 2011). Antioxidants are substances that, when used in low concentrations, can reduce the oxidation of macromolecules, including lipids, through the inhibition of free radicals or by reducing chelating metal ions (Echegaray et al. 2021). In a previous study, Kim et al. (2016) evidenced the FRSA properties of SCG extracts (ethanol > aqueous, in concentration dependence) obtained by percolation extraction. Also, Bouhlal et al. (2020) reported FRSA properties of the aqueous-ethanol extract obtained from SCG (C. arabica and C. Robusta) using Soxhlet as extraction method. While Okur et al. (2021) demonstrated that SCG methanol-aqueous extract (C. arabica) obtained by percolation (solvent-solid ratio of 8:2), high hydrostatic and ultrasound-assisted extraction exert FRSA and FRAP activity. In addition, García-Larez et al. (2021) reported that SCG extracts (aqueous, ethanol, and aqueous-ethanol) showed antiradical activity (FRSA and RCSA), as well as reducing power properties (RPA and FRAP), which was associated with the presence of phenolic compounds like phenolic acids and flavonoids.
Regard biotechnological extraction methods, Palomino-Garcia et al. (2015) reported that coffee residues extracts obtained by solid-state fermentation using Penicillium purpurogenum, exert FRSA (C. robusta: husk > pulp), which was associated with an enhancement of phenolic compounds like caffeoylquinic acid, caffeic acid, and rutin. Also, Moreira et al. (2018) reported that coffee residue methanol-aqueous extract (C. arabica; husk and pulp; solvent-solid ratio of 7:3) obtained by solid-state fermentation using Rhodotorula mucilaginosa, exert antiradical activity (FRSA). While Han et al. (2021) demonstrated that SCG extract obtained by submerged-state fermentation using Cordyceps sinensis, exert FRSA, RCSA and FRAP activity. Although there is limited information on the use of submerged-culture fermentation using P. ostreatus as potential alternative to extract bioactive compounds from coffee residues, in our study the potential use of submerged fermentation with P. ostreatus for the recovery of antioxidant compounds, which could be used as preservative additives in the food industry.
According to our study a positive correlation was found between phytochemical content and antioxidant properties, mainly for reducing power activity (r2 = 0.90 approx.). In a previous study, it has also been demonstrated the relationship of phenolic compounds and antioxidant activity (r2 > 0.90) of coffee brews (Miłek et al. 2021). Also, it has been demonstrated a high correlation (r2 > 0.90) between phenolic acids with antirradical properties of unprocessed a processed coffee (Pérez-Hernández et al. 2012).
According to the findings of antioxidant activity obtained in our study, the aqueous extract obtained from SCG fermented with P. ostreatus could be used as an additive to extend the shelf life of fresh meat and meat products.
Lipid oxidation inhibition
Lipid oxidation is considered one of the main factors that affect the sensory and nutritional quality in food products, and the methods used to determine lipid oxidation level measured the lipid oxidation´s primary or secondary products. For example, primary oxidation products can be measured using peroxide, iodine, and conjugate dienes measurements, while the 2-thiobarbituric acid reactive substances method (TBARS) is highly used as indicator of secondary oxidation products like malonaldehyde (MDA) (Kim et al. 2016).
In a previous study, Jully et al. (2016) reported that the inclusion of the aqueous extract from SCG obtained by percolation, reduced lipid oxidation in frozen cooked pork patties during storage (-18 °C for three months). In addition, Kim et al. (2016) demonstrated the lipid oxidation inhibition properties of SCG extracts (ethanol > aqueous, in concentration dependence) obtained by percolation extraction, in an oil emulsion model system during storage period, at 37 °C for 72 h. It has been also demonstrated a similar effect against lipid oxidation of raw meat homogenates treated with SCG, during storage at 37 °C for 12 h, as well as in cooked chicken patties incorporated with SCG extracts stored at 4 °C for 5 d. Regard biotechnological extraction methods, Corrêa et al. (2015) reported that an ethanolic-aqueous extract (solvent-solid ratio 7:3) obtained by submerged fermentation using Pleurotus ostreatoroseus, exert lipid oxidation inhibition in brain pork tissue homogenates stored at 37 °C for 1 h. However, there is still no information on the use of extracts obtained by fungal fermentation, on oxidative stability in meat products. Therefore, our study confirmed that the extract obtained by fungal fermentation using P. ostreatus, exerted activity against the formation of lipid oxidation secondary products.
Correlation between metabolites and antioxidant properties
According to the findings, Pearson´s correlation and principal component analysis demonstrated a positive correlation between metabolites of aqueous extract obtained from SCF fermented with P. ostreatus, and their biological properties. It has been extensively reported that metabolites contributing to antiradical and reducing power ability of natural extracts, however, this positive correlation can be affected by the presence or absence of other compounds, which can improve or interfere with their bioactivity (Terpinc et al. 2012, Moazzen et al. 2022).
Conclusions
This investigation demonstrated that the aqueous extract obtained by submerged-state fermentation of coffee residues (SCG, spent coffee grounds) using P. ostreatus, increased the release of phytochemicals (carbohydrates and phenolic compounds), as well the antioxidant properties (antiradical and reducing power), in comparison to the unfermented extract. In addition, the inclusion of SCG extract on pork meat homogenates reduced the lipid oxidation during storage. Therefore, extracts obtained by fungal fermentation process of agro-industrial wastes using Pleurotus spp., could be used as a potential strategy to obtain natural additives.











 nueva página del texto (beta)
nueva página del texto (beta)