Introduction
James Hope described a sinus of Valsalva aneurysm (SVA) case, ruptured into the right ventricle in 1839, considered then a congenital anomaly. Most of the unruptured cases of SVA remain asymptomatic and undetected, although confused with other pathologies such as pericardial cysts.1 Most of these aneurysms are generally silent; however, autopsy studies of 8,138 individuals suggested a prevalence of 0.09% in the general population.2 The SVA is usually congenital in etiology, but other acquired etiologies include atherosclerosis, syphilis, Marfan’s syndrome, or infective endocarditis has been described. Pathophysiologically, SVA results from the Valsalva sinus’s dilation, from a separation between the aortic media and annulus fibrosus due to elastin and collagen deficit causing wall stiffening, more frequently affecting the right sinus. The diagnosis includes image multimodalities with a size range of 20 to more than 50 mm in different series, usually starting with transthoracic Doppler-echocardiography, later confirming with transesophageal one, contrast computed tomography, magnetic resonance, and catheter-based angiography. The true history of SVA is not completely clear, but the rupture of SVA is a possibly preventable fatal complication.
We discuss an unusual unruptured sinus of Valsalva aneurysm due to the scarcely available literature on this presentation mode, with special descriptive and evocative cardiac imaging views with an innovative corrective surgical technique.
Case presentation
We received a 72-year-old athletic male with no significant past medical condition, referred for evaluation of a new systolic pulmonary focus murmur. The patient reported no symptoms and performed daily jogging. Chest X-ray was read as normal (Figure 1).
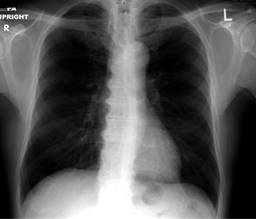
Figure 1: Anteroposterior view of chest radiograph, note that it is not able to detect the huge problem.
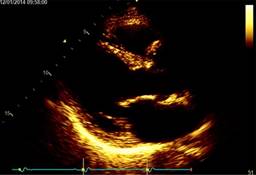
On physical examination, the blood pressure was 130/78 mmHg, heart rate 78 bpm, and no respiratory distress with a grade II/VI systolic ejection on the left upper sternal border, with normal pulses, without pericardial rub, jugular dilatation or paradoxical pulse. The echocardiogram revealed a mildly reduced Simpson’s rule calculated ejection fraction of 46% and inferolateral mild hypokinesis. The valvular examination revealed mild aortic and mitral insufficiency, dilated ascending aorta dimension at the sinus of Valsalva level, severe aneurysmal dilatation (2.5 × 4.6 mm) of a non-ruptured right sinus of Valsalva with right ventricular outflow tract compression and increased flow velocity in the pulmonary valve (Figure 2).
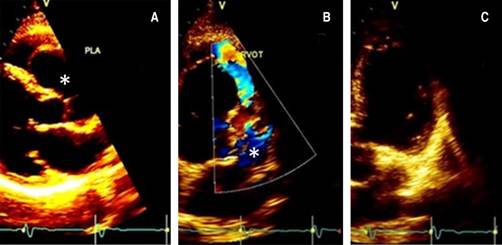
Figure 2: Doppler-echo pre-treatment; the asterisk shows the aneurysm in long (A) and short (B) parasternal and apical (C) view.
The CT angiography confirmed the echocardiogram findings and suggested a small right coronary artery (RCA), not shown in our Figures, and a normal left anterior descending coronary artery with compression of the right ventricular outflow tract (Figure 3).
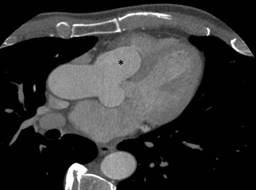
Figure 3: A chest computed tomography-sagittal view showing dilation of the aortic root and expansion of the aneurysm (asterisk) into the right ventricle.
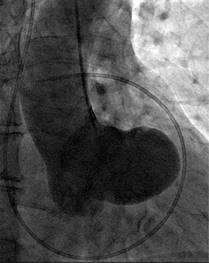
The cardiac catheterization presented normal coronaries with a small right coronary artery. The left ventricular angiogram presented inferior mild hypokinesia, and the aortogram revealed an abnormal appearance which we described as «a boot in the heart», produced by the severely dilated non-ruptured right sinus of Valsalva without fistulous tracts (Figure 4).
The patient had repair of the non-ruptured right coronary sinus of the Valsalva aneurysm with a J-shaped mini sternotomy through the fourth intercostal space, exposing the ascending aorta followed by arterio-venous femoral cannulation for cardiopulmonary bypass and coronary sinus cannulation under transesophageal echocardiogram assistance. The right superior pulmonary vein was used for decompression and venting the heart, later exploring the anterior aorta and valve through a hockey stick incision to examine the aneurysm. With Prolene 5-0, concentric purse strings were applied from the lower dome of the aneurysm and up until the defect’s obliteration.
After measuring the aortic sinus defect, a piece of autologous pericardium was meticulously prepared and tailored according to the measurements and shape of the defect, carefully suturing the pericardial patch continuously to the aortic defect with Prolene 5-0, confirming no-insufficiency of the valve finally weaning the patient from cardiopulmonary bypass as usual. After surgery, the patient recovered with minimal analgesic drugs requirement, removing the chest tube and the pacing wires on the second postoperative day and discharged to home on the fourth postoperative day, without complications at follow up, when he had a significantly modified sinus of Valsalva aspect (Figure 5).
Discussion
The SVA are small dilations in the aortic wall just above any of the three aortic valve cusps between the aortic valve annulus and the sinotubular ridge, usually secondary to infection or trauma that implies the aortic media separation from the annulus fibrosus,3 most often occurring in the right coronary or noncoronary sinus. These aneurysms are thin walled-outpouchings prone to rupture. When this occurs, they can form a fistulous connection with one of the heart chambers, causing symptoms. The patients may be asymptomatic or may have ischemic events, cardiac rhythm abnormalities, and embolic events. In ruptured aneurysms, symptoms may include dyspnea, chest pain, fatigue, peripheral edema, and, in the worst scenario, sudden cardiac death or tamponade.
Symptoms can be gradual or acute, sometimes associated with other cardiac abnormalities, such as ventricular septal defects and aortic valve dysfunction. The physical examination may disclose a continuous mechanical-sounding murmur similar to the patent ductus arteriosus. The electrocardiogram is usually normal in unruptured aneurysms except if the aneurysm compresses the atrioventricular node, perhaps causing conduction disturbances.3 In most cases, for the diagnosis of SVA, transthoracic and transesophageal echocardiography can provide adequate identification and description of the size and severity, as described in our referred case reports, either with2,3 or without cardiac catheterization1 or echocardiography and cardiac catheterization.4 Chest computerized tomography (CCT), in addition to magnetic resonance, can also be used as part of the workup for SVA identification, and in other cases where the clinical suspicion is high but not seen in the previous images, angiography is an acceptable option. Nowadays, the only accepted and recommended treatment is surgery. The first SVA repair cases were described in the 1950s,3 with the surgical treatment usually indicated in the presence of rupture, compression of adjacent structures, and significant dilation or aortic regurgitation.2 Other surgery indications include non-ruptured SVA-producing malignant arrhythmia, obstructing coronary ostia or ventricular outflow tract, or infection.4,5 The operative mortality after an SVA surgical repair is relatively low, with 80 to 97% alive patients at five and ten years, respectively. The presented case has the importance of being one of the few cases detected before rupture, possessing evocative and descriptive imaging, and considered perhaps, to our knowledge, if not the first, one of the few nonruptured cases corrected with a minimally invasive surgical procedure.
Conclusions
This case presents a characteristic shape that may rupture with a fatal outcome.
The early diagnosis and treatment may be life-saving. The image diagnosis usually starts with transthoracic echocardiography, later confirmed and detailed by contrast computed tomography, magnetic resonance and catheter-based angiography.











 nueva página del texto (beta)
nueva página del texto (beta)